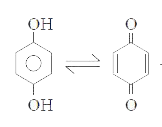
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the half cell, +2H^(+)2e^(-).E^(0)= 1.30 V. At pH = 2, elec...
Text Solution
|
- For the half cell At pH=2, the electrode potential is
Text Solution
|
- At pH = 2, E(("Quinhydrone"))^(@) = 1.30 V, E("Quinhydrone") will be :
Text Solution
|
- For the half cell At pH=2. Electrod potential is :
Text Solution
|
- For the half cell At pH = 3 electrode potential is
Text Solution
|
- The standard electrode potential of the half cells are given below. Zn...
Text Solution
|
- For the half cell, +2H^(+)2e^(-).E^(0)= 1.30 V. At pH = 2, elec...
Text Solution
|
- The standard electrode potentials of the two half cells are given belo...
Text Solution
|
- The standard electrode potential of the htwo half cells are given belo...
Text Solution
|