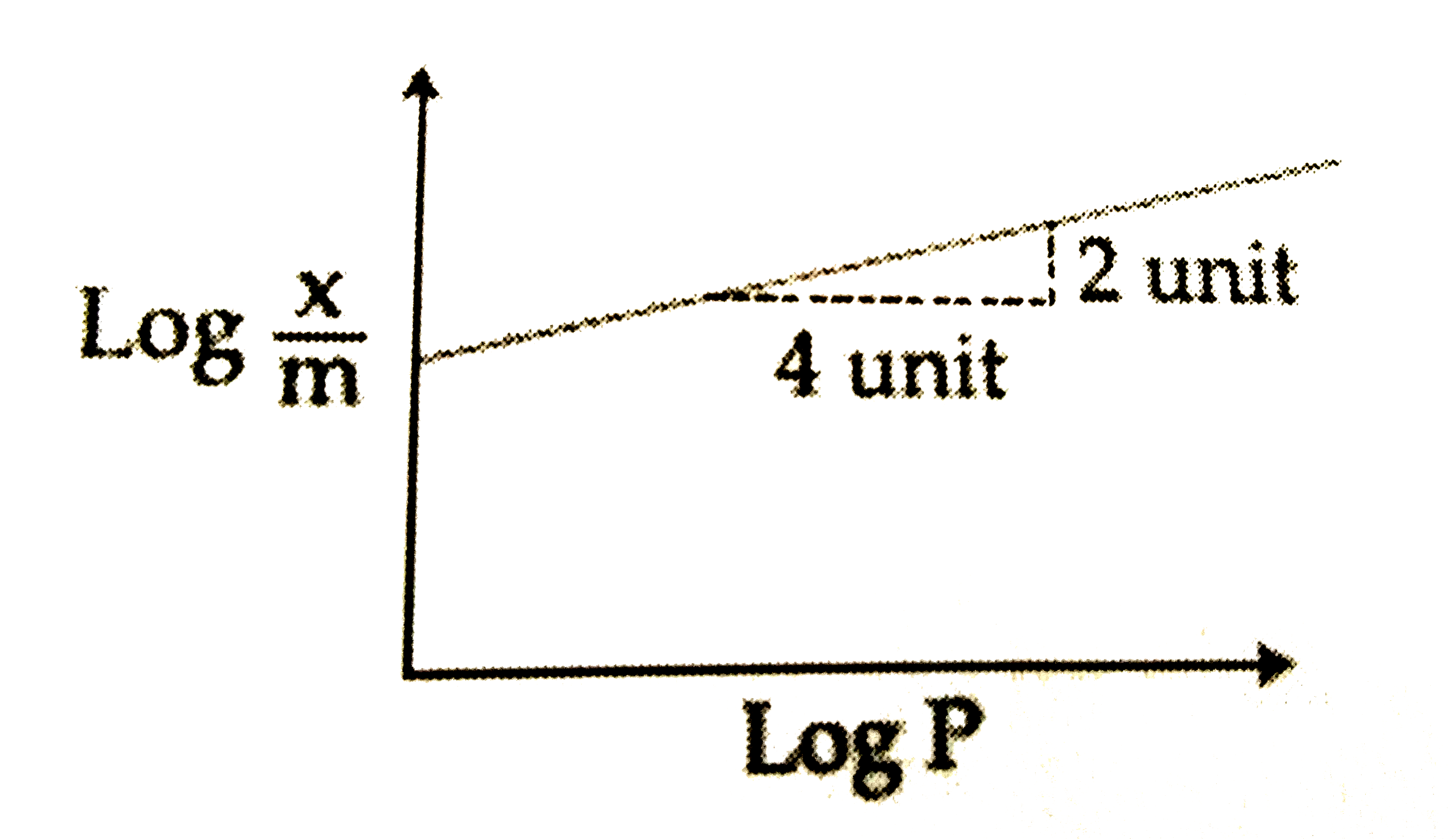A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Adsorption of a gas follows Freundlich adsorption isotherm. In the giv...
Text Solution
|
- Adsorption of a gas follows Freundlich adsorption isotherm. In the giv...
Text Solution
|
- Adsorption of a gas on a surface follows Freundlich adsorption isother...
Text Solution
|
- Adsorption of a gas follows Freundlich adsorption isotherm. In the giv...
Text Solution
|
- If the value of 1/n is equal to 1 in Freundlich adsorption isotherm, t...
Text Solution
|
- Adsorption of gas follows Freundlich adsorption isotherm. If x is the ...
Text Solution
|
- Adsorption of a gas follows Freundlich adsorption isotherm. In the giv...
Text Solution
|
- Adsorption of a gas follows Freundlich adsorption isotherm. x is the m...
Text Solution
|
- Adsorptionof a gas follows Freundlich adsorption isotherm. x is the ma...
Text Solution
|