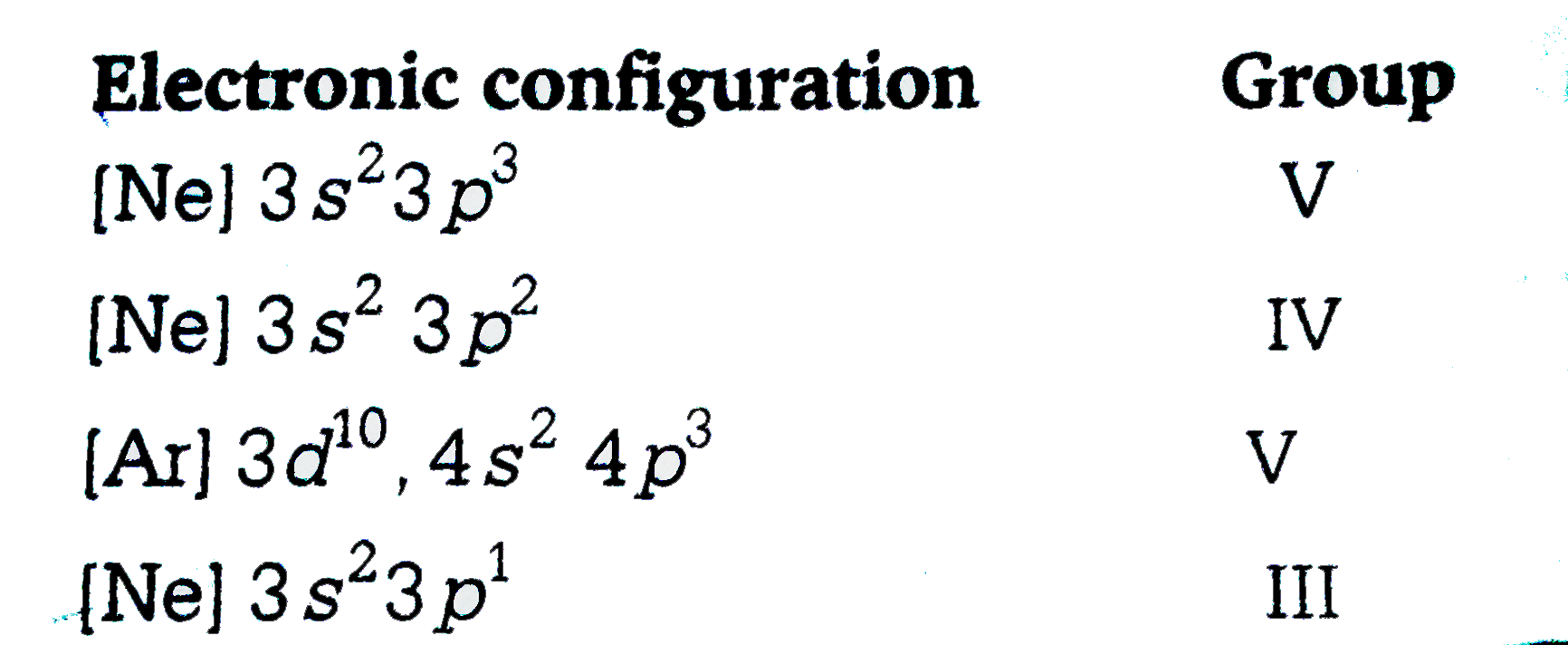A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-CHEMICAL PERIODICITY-Exercise
- The correct decreasing order of ionic size among the following species...
Text Solution
|
- Which one of the following arrangements represents the correct order o...
Text Solution
|
- Which one of the following elements has the highest ionisation energy?
Text Solution
|
- Which one of the elements with the following outer orbital configurati...
Text Solution
|
- Which of the following oxides is not expected to react with sodium hyd...
Text Solution
|
- The correct of decreasing second ionisation enthalpy of Ti(22),V(23),C...
Text Solution
|
- Which of the following electrons configruations of an atom has the low...
Text Solution
|
- The correct order of the size is
Text Solution
|
- Ionic radii are
Text Solution
|
- The ions O^(2-), F^(-), Na^(+), Mg^(2+), and A1^(3+) are isolectronic....
Text Solution
|
- Which of the following order is wrong-
Text Solution
|
- An atom has electronic configuration 1s^(2) 2s^(2) 2p^(6) 3s^(2) 3P^(6...
Text Solution
|
- Correct order of 1st ionisationpotential (IP) among following elements...
Text Solution
|
- The first ionisation potentials (eV) of Be and B respectively are
Text Solution
|
- In the crystals of which of the following ionic compounds would you ex...
Text Solution
|
- Which one of the following ions will be smalllest in size?
Text Solution
|
- The element with atomic number Z = 118 will be categorised as a:
Text Solution
|
- The electronic configuration of an element is 1s^(2)2s^(2)2p^(6),3s^(2...
Text Solution
|
- Among the following, the one which is the most basic is
Text Solution
|
- One of the characteristic propertise of non-metals is that they
Text Solution
|