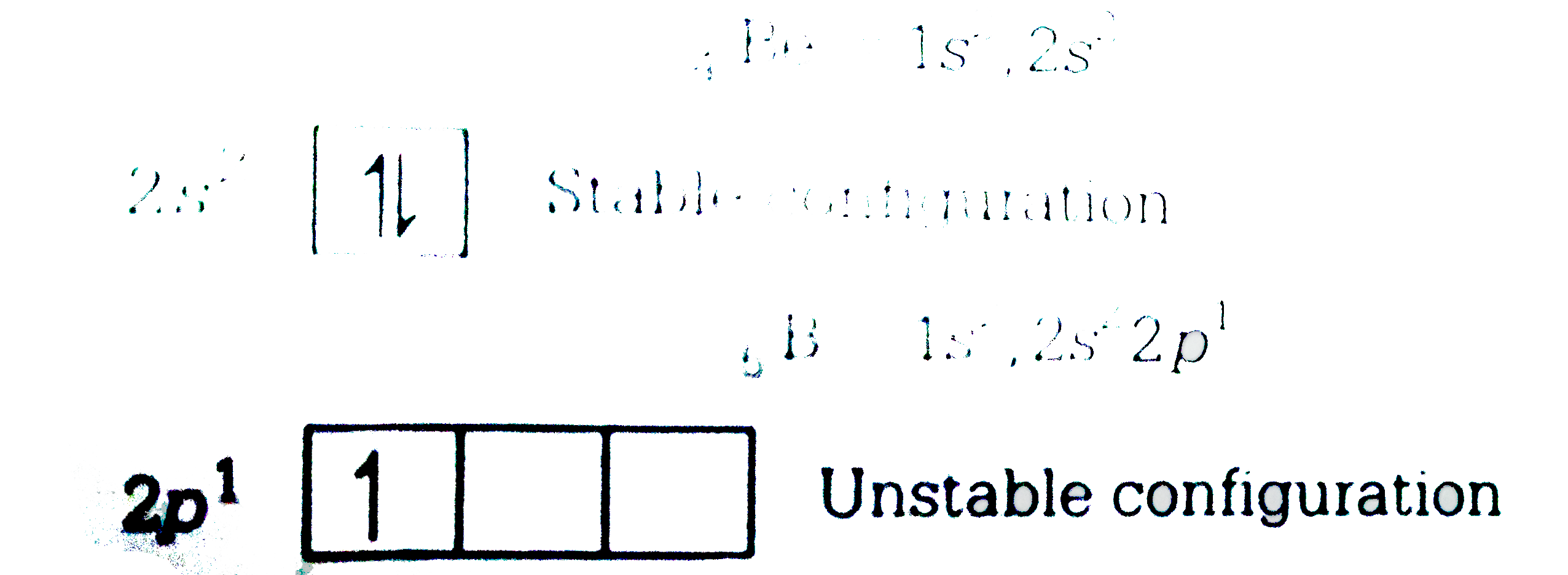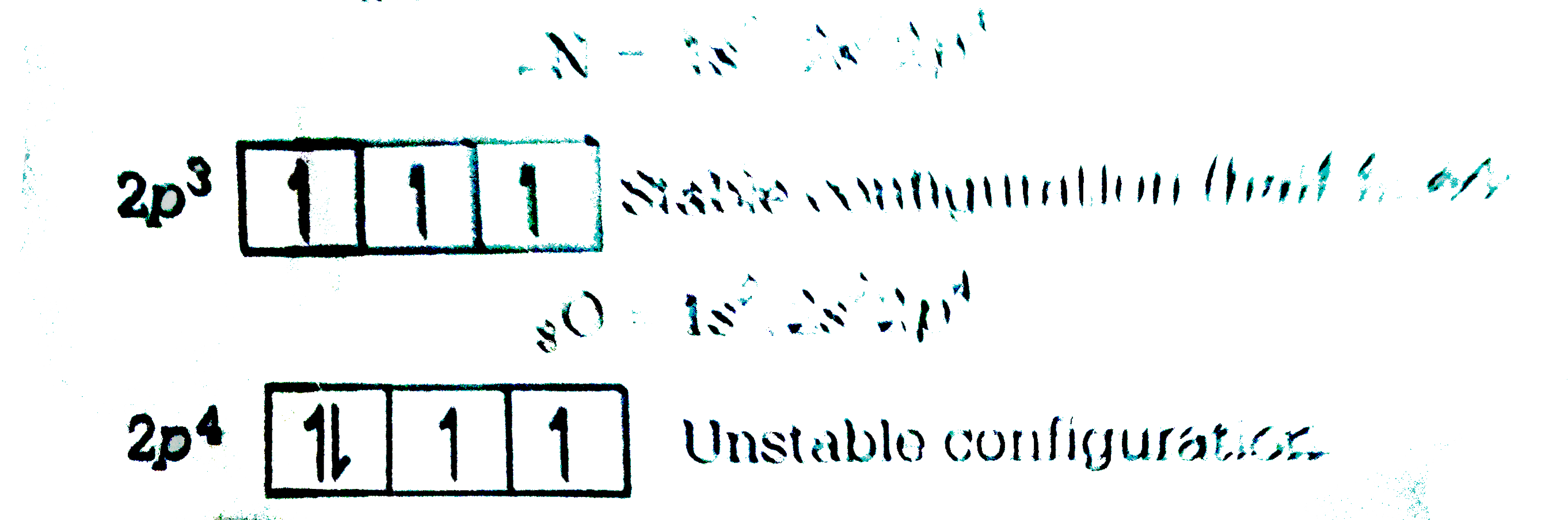A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-CHEMICAL PERIODICITY-Exercise
- The ions O^(2-), F^(-), Na^(+), Mg^(2+), and A1^(3+) are isolectronic....
Text Solution
|
- Which of the following order is wrong-
Text Solution
|
- An atom has electronic configuration 1s^(2) 2s^(2) 2p^(6) 3s^(2) 3P^(6...
Text Solution
|
- Correct order of 1st ionisationpotential (IP) among following elements...
Text Solution
|
- The first ionisation potentials (eV) of Be and B respectively are
Text Solution
|
- In the crystals of which of the following ionic compounds would you ex...
Text Solution
|
- Which one of the following ions will be smalllest in size?
Text Solution
|
- The element with atomic number Z = 118 will be categorised as a:
Text Solution
|
- The electronic configuration of an element is 1s^(2)2s^(2)2p^(6),3s^(2...
Text Solution
|
- Among the following, the one which is the most basic is
Text Solution
|
- One of the characteristic propertise of non-metals is that they
Text Solution
|
- A sudden large jump between the values of second and third ionisation ...
Text Solution
|
- In the periodic table from left to right in a period, the atomic volum...
Text Solution
|
- If the atomic number of an element is 33, it will be placed in the per...
Text Solution
|
- Na^(+), Mg^(2+), Al^(3+), and Si^(4+) are isoelectronic ions. Their io...
Text Solution
|
- One would expect proton to have very large
Text Solution
|
- Which of the following set has the strongest tendency to form anions?
Text Solution
|
- The ionisation of hydrogen atom would give rise to
Text Solution
|
- In the periodic table, with the increase in atomic number the metallic...
Text Solution
|
- The electronic configuration of four elements are given below. Which e...
Text Solution
|