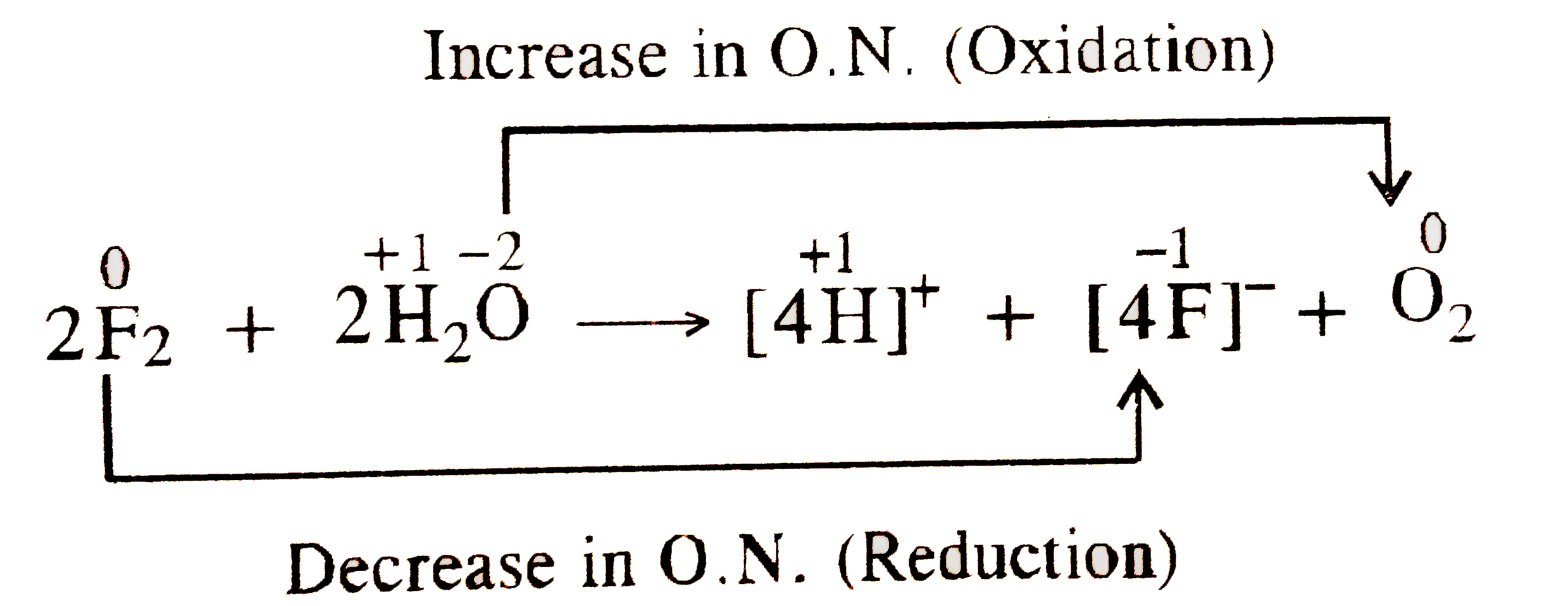Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the reaction of water with F(2) and suggest in terms of oxida...
Text Solution
|
- DEFINITION OF OXIDATION AND REDUCTION, OXIDISING AND REDUCING AGENT
Text Solution
|
- Consider the reacton of water with F(2) and suggest, in terms of oxida...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Justify that the reaction : 2Cu(2)+Cu(2)Srarr6Cu+SO(2) is a redox ...
Text Solution
|
- Consider the reaction of water with F(2) and suggest in terms of oxida...
Text Solution
|
- Consider the reaction of water with F(2) and suggest , in terms of oxi...
Text Solution
|
- F(2) के साथ जल की अभिक्रिया में ऑक्सीकरण तथा अपचयन के पदों पर विचार की...
Text Solution
|
- ऑक्सीकरण, अपचयन, ऑक्सीकारक तथा अपचायक को ऑक्सीकरण संख्या के पदों में ...
Text Solution
|