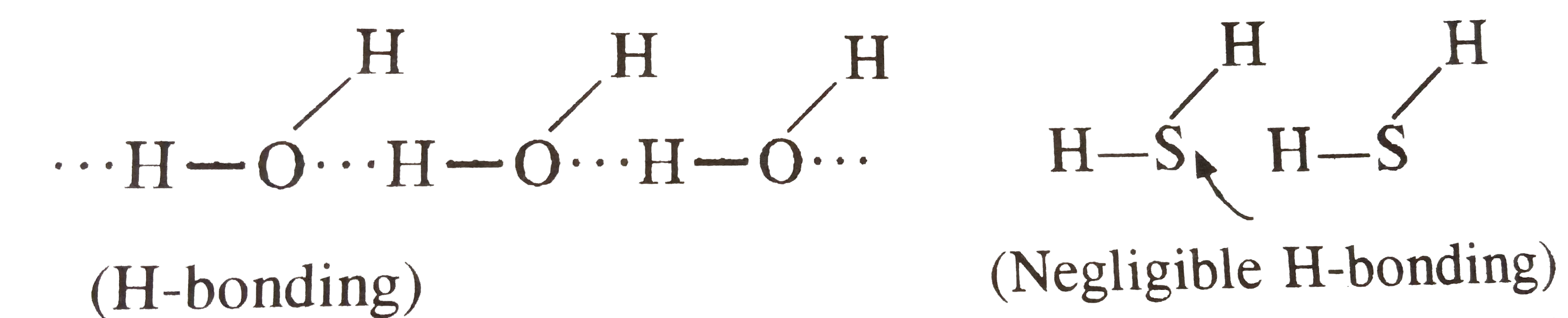Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain why water has high melting and boiling points as compared to H...
Text Solution
|
- Explain why water has high boling and melting points are compared to H...
Text Solution
|
- Explain why water has high melting and boiling points as compared to H...
Text Solution
|
- स्पष्ट कीजिए कि आयनिक यौगिक - उच्च क्वथनांक तथा गलनांक क्यों दर्श...
Text Solution
|
- सामान्य ताप पर H(2)O द्रव है जबकि H(2)S गैस है, क्यों? जल का कवथनांक उ...
Text Solution
|
- NH3 has exceptionally high melting point and boiling point as compared...
Text Solution
|
- NH3 has exceptionally high melting point and boiling point as compared...
Text Solution
|
- Why does water has a high boiling point and a high melting point as co...
Text Solution
|
- H(2)S Melting point and boiling point of water is higher than that of ...
Text Solution
|