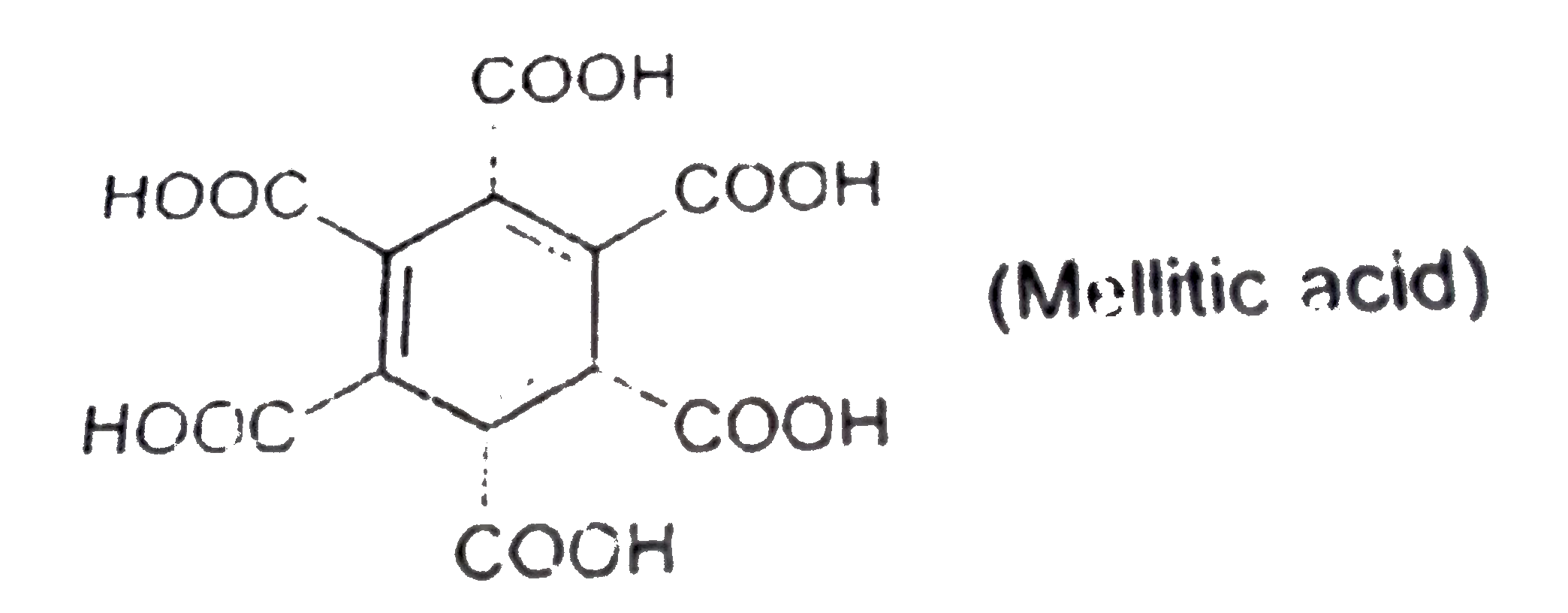
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise Exercise-1 Part-II : ONLY ONE OPTION CORRECT TYPE Section (D) : Chemical properties of elements (AI,Sn,Pb)|8 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise Exercise-1 Part-II : ONLY ONE OPTION CORRECT TYPE Section (E) : Hydrides|8 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise Exercise-1 Part-II : ONLY ONE OPTION CORRECT TYPE Section (B) : Isolation of elements (B,AI,C,Si)|7 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P-BLOCK ELEMENT (BORON AND CARBON FAMILY)-Exercise-1 Part-II : ONLY ONE OPTION CORRECT TYPE Section (C) : Chemical properties of elements (B,C,Si)
- Producer gas is a mixture of
Text Solution
|
- Silicon react with hot solution of NaOH forming:
Text Solution
|
- Concentrated HNO(3) turns yellow in sun light Explain ?
Text Solution
|
- When steam is passed through red hot coke:
Text Solution
|
- Amorphous boron on burning in air forms
Text Solution
|
- Carbon and silicon belong to group IV. The maximum coordination number...
Text Solution
|
- Boron form covalent compound due to
Text Solution
|