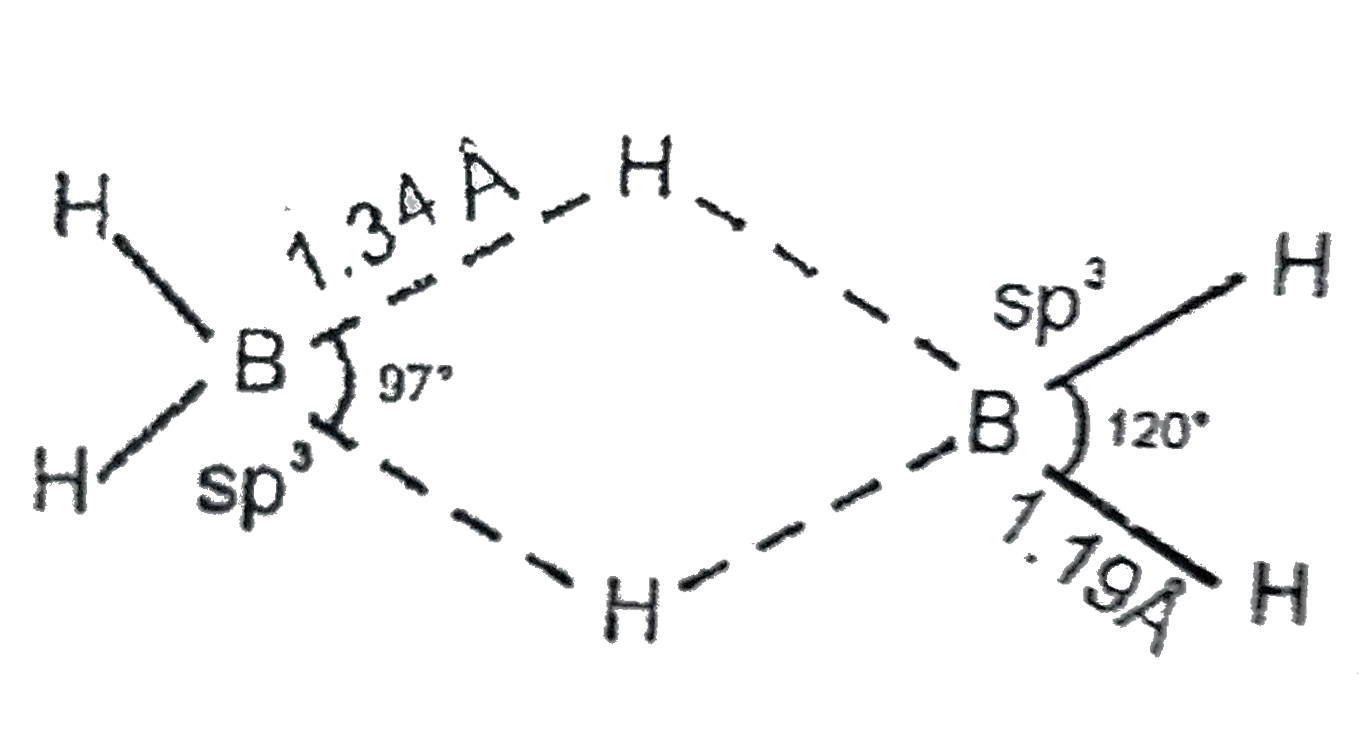
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise Exercise-1 Part-II : ONLY ONE OPTION CORRECT TYPE Section (F) : Oxides, Oxyacids & Hydroxides ; Borax|10 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise Exercise-1 Part-II : ONLY ONE OPTION CORRECT TYPE Section (G) : Halides, Alums & Other metal salts|15 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise Exercise-1 Part-II : ONLY ONE OPTION CORRECT TYPE Section (D) : Chemical properties of elements (AI,Sn,Pb)|8 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P-BLOCK ELEMENT (BORON AND CARBON FAMILY)-Exercise-1 Part-II : ONLY ONE OPTION CORRECT TYPE Section (E) : Hydrides
- From B(2)H(6), all the following can be prepared except
Text Solution
|
- The product obtained in the reaction of diborane with excess of ammoni...
Text Solution
|
- Diborane reacts with water to form:
Text Solution
|
- In diborane, the two H-B-H angles are nearly
Text Solution
|
- In the reaction: BF(3)+3LiBH(4)to3LiF+X, X is
Text Solution
|
- Diborane upon hydrolysis gives
Text Solution
|
- Which of the statement is true for the above sequence of reactions?
Text Solution
|
- Which of the following is known as inorganic benzene
Text Solution
|