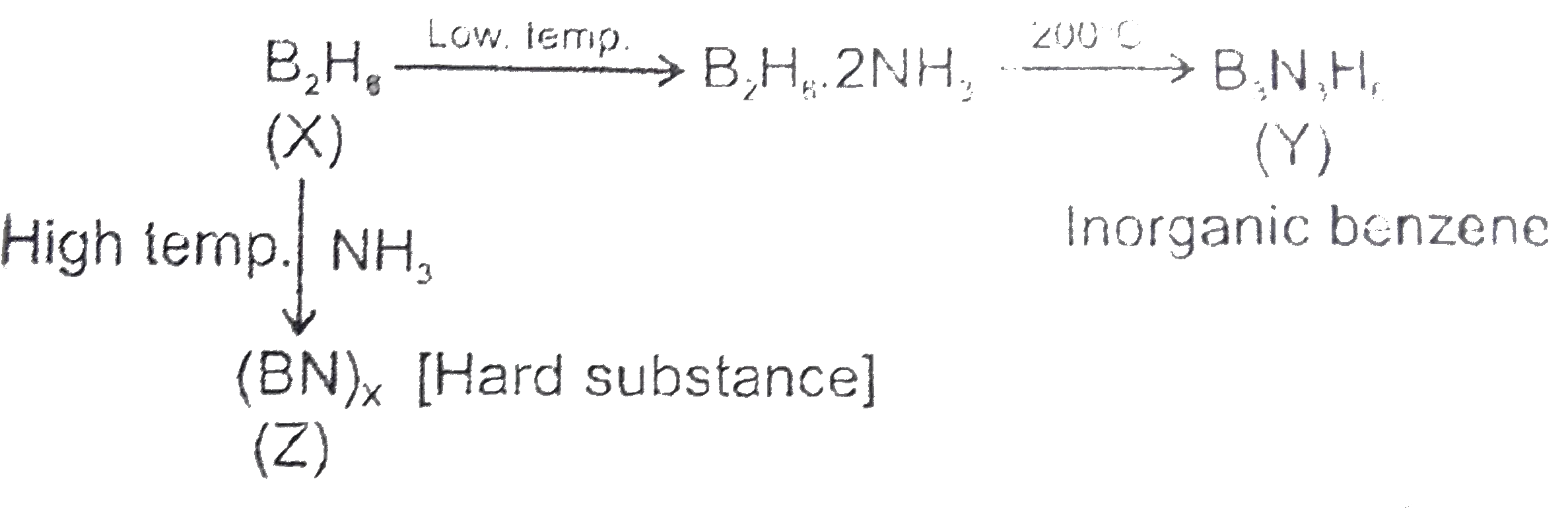
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise SECTION-3|6 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise SECTION-4|3 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise SECTION-1|7 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P-BLOCK ELEMENT (BORON AND CARBON FAMILY)-SECTION-2
- Which of the following are the ores of Boron
Text Solution
|
- When as inorganic compound X having electron dificient bounding (banan...
Text Solution
|
- Which of the following statement is/are correct ?
Text Solution
|
- Which of the following is/are true about silicones.
Text Solution
|
- Cation exchanger zeolite Na(12)Al(12)Si(17)O(58).27H(2)O can exchange ...
Text Solution
|