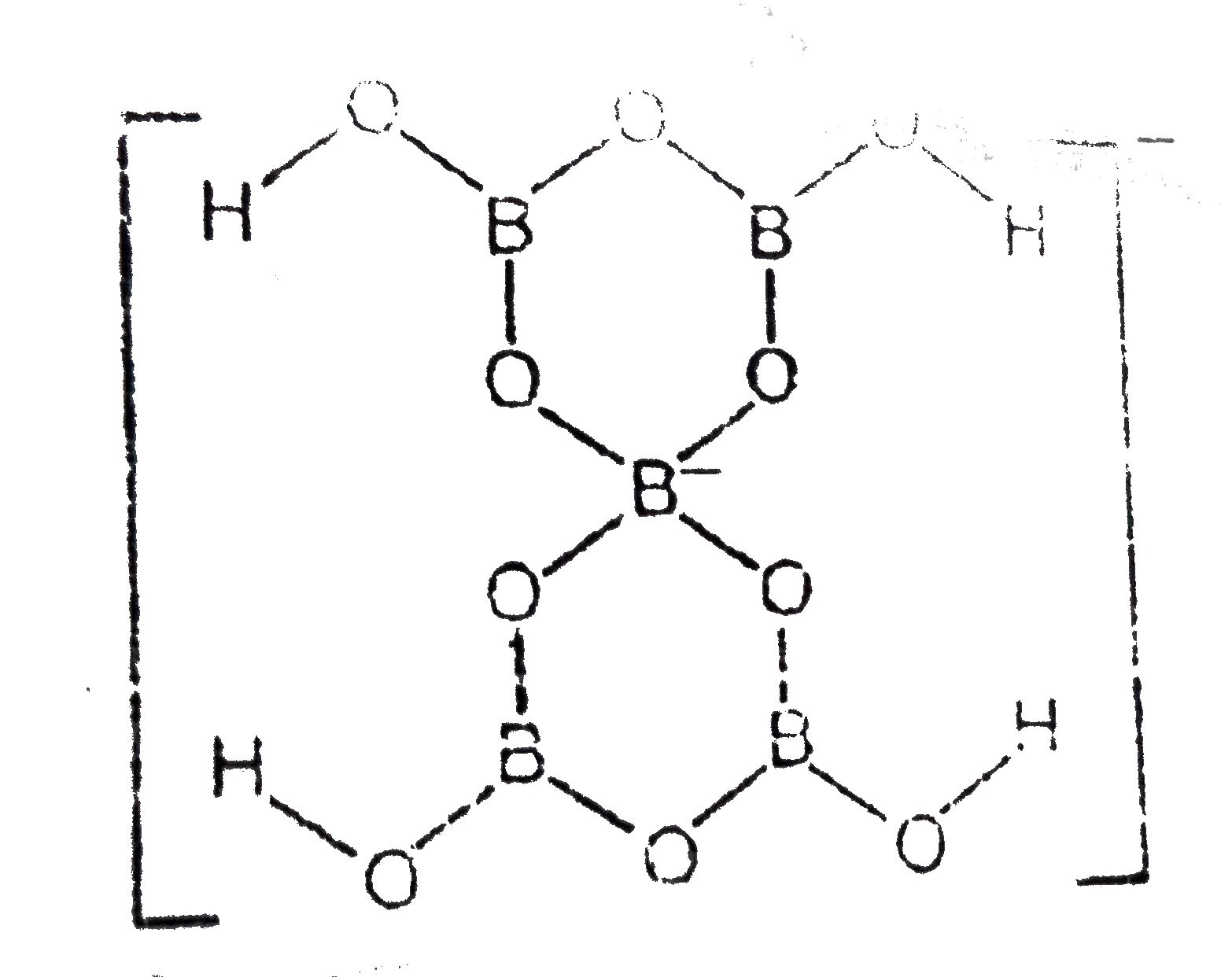
Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise SECTION-4|3 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - I (NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC))|14 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE|Exercise SECTION-2|5 VideosP BLOCK ELEMENTS
RESONANCE|Exercise PART -II|24 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-P-BLOCK ELEMENT (BORON AND CARBON FAMILY)-SECTION-3
- How many of the following parameters/properties are greater for diamon...
Text Solution
|
- Na(2)B(4)O(7)overset(Delta)toNaBO(2)+B(2)O(3) NaBO(2)+H(2)O(2)+H(2)O...
Text Solution
|
- A Boron mineral have pentaborate anion whose molecular formula is [B(5...
Text Solution
|
- Consider the following sequence of reactions: B(2)O(3)+CaF(2)+H(2)S...
Text Solution
|
- How many of the following statement are correct ? (1) CF(4) can be ...
Text Solution
|
- How many of the following may react with SiCl(4) to produce a non-pola...
Text Solution
|