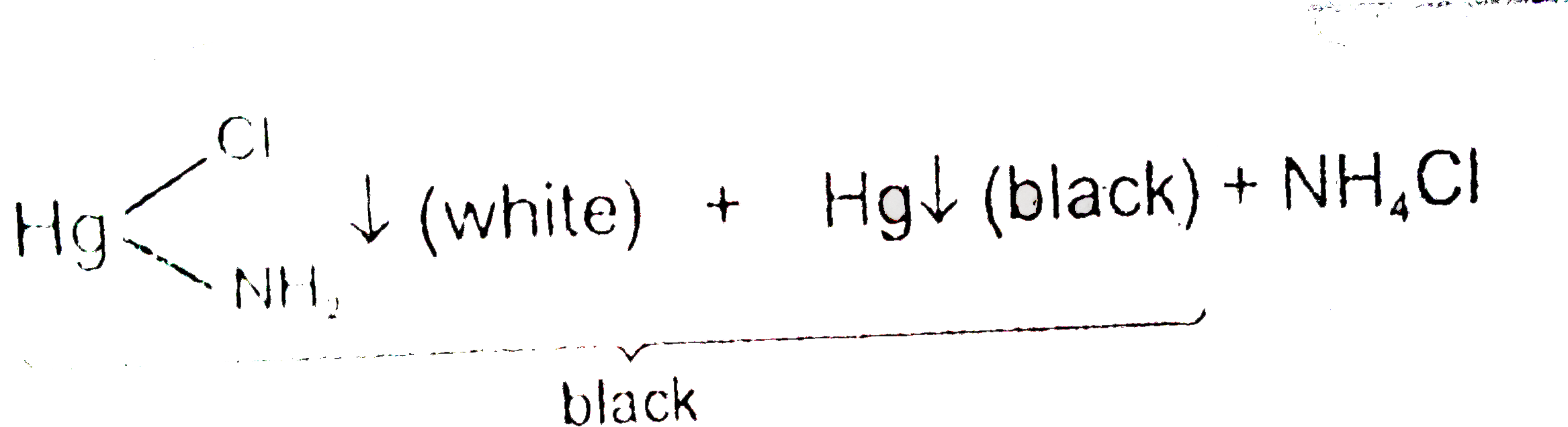A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- To a solution of a substance, gradual addition of ammonium hydroxide r...
Text Solution
|
- To a solution of a substance gradiual addition of ammonium hydroxide...
Text Solution
|
- Al^(3+),Fe^(3+),Zn^(2+) and Ni^(2+) ions are present in an acidic solu...
Text Solution
|
- To a solution of a substance, gradual addition of ammonium hydroxide r...
Text Solution
|
- Statement-1:NO yellow precipitate is formed when an excess of a more c...
Text Solution
|
- To a solution of a substance, gradual addition of ammonium hydroxide r...
Text Solution
|
- A white precipitate is obtained when a solution is diluted with H(2)O ...
Text Solution
|
- To a solution of a substance, gradual addition of ammonium hydroxide r...
Text Solution
|
- On addition of aqueous NaOH to a salt solution, a white gelatinous pre...
Text Solution
|