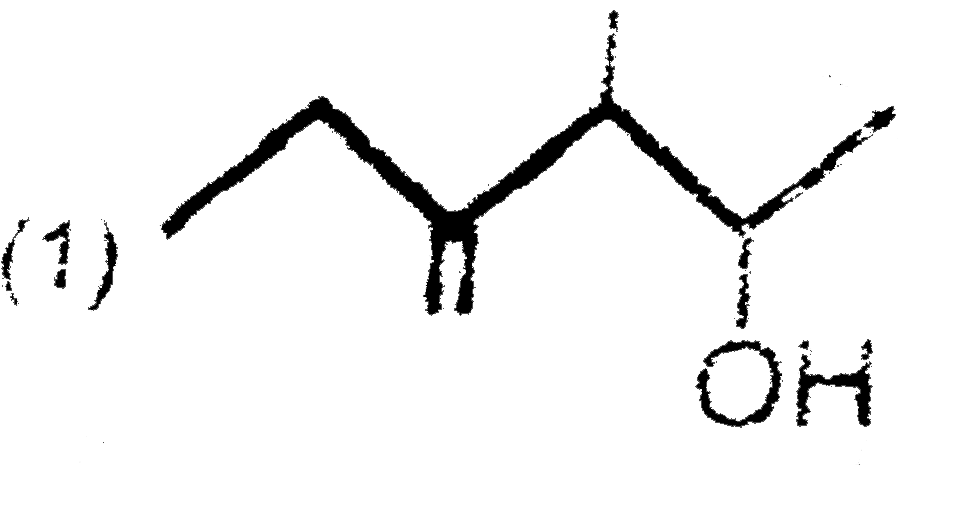
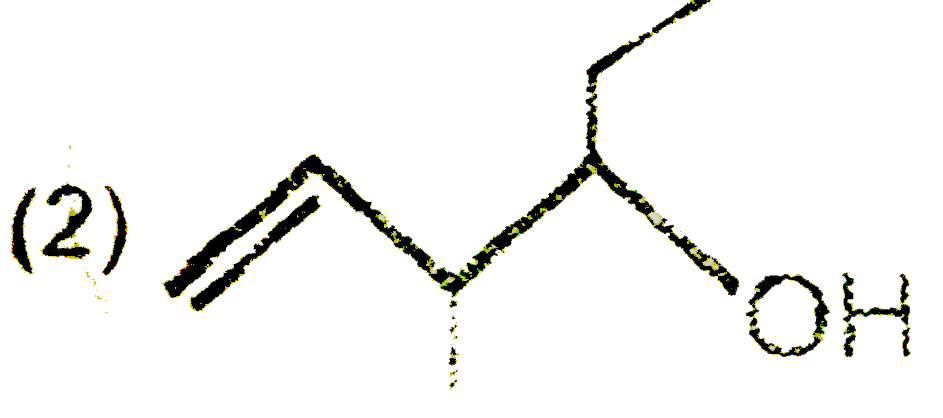
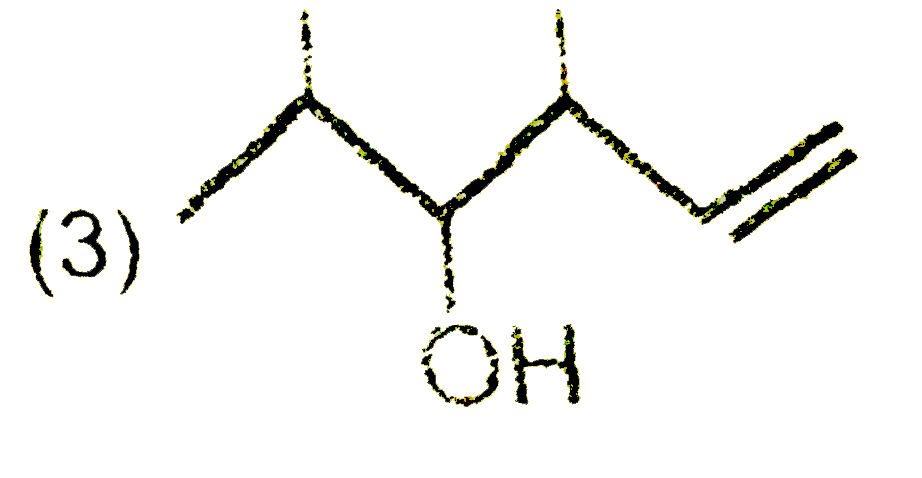
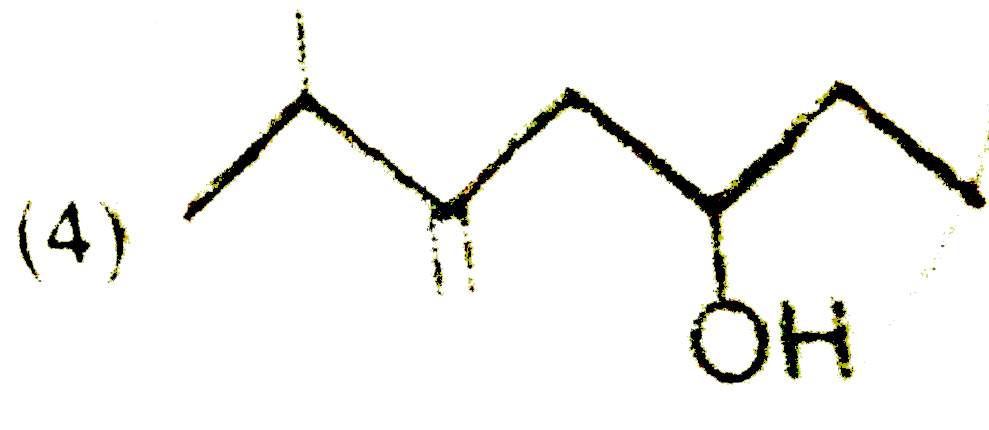
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE|Exercise Advanced Level Problems Part-2 : Practice test-2|22 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE|Exercise Advanced Level Problems Part-3|18 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE|Exercise Exercise-3 Part-2|8 VideosIONIC EQUILIBRIUM
RESONANCE|Exercise partIII one or more than one options correct type|7 VideosMETALLURGY
RESONANCE|Exercise INORGANIC CHEMISTRY(Metallurgy)|42 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM -Advanced Level Problems Part-1 : Practice test-1
- The IUPAC name of the compound shown below is
Text Solution
|
- The IUPAC name of compound CH(3)-C(CH(3))(2)-CH(2)-CH=CH(2) is
Text Solution
|
- What is the structure of 4-Methylhex-5-en-3-ol.
Text Solution
|
- A compound having straight chain of five carbon atoms has one ketone g...
Text Solution
|
- What is the IUPAC name of
Text Solution
|
- What is the IUPAC name of compound is:
Text Solution
|
- The IUPAC name of CH(2)-CH(2)-undersetunderset(CH(3))(|)(N)-CH(2)-CH(3...
Text Solution
|
- In the given formula G is an unknown group. What will be the gro...
Text Solution
|
- IUPAC name of , is
Text Solution
|
- Correct IUPAC name of given ester is: CH(3)-undersetunderset(Br)(|)(...
Text Solution
|
- Relation between Ethyl benzenecarboxylate and phenyl propanoate is:
Text Solution
|
- The correct IUPAC name of the compound , is:
Text Solution
|
- Which of the following pair of compounds is not functional isomers?
Text Solution
|
- are related as:
Text Solution
|
- Which of the following pairs of structures do not represent isomers?
Text Solution
|
- Total number of structural isomers possible from molecular formula C(8...
Text Solution
|
- Total number of position isomers of trimethyl cyclohexane are:
Text Solution
|
- How many 1^(@) amines are possible with molecular formula C(4)H(11)N ...
Text Solution
|
- Hybridisation of carbon atoms present in the smallest ester are:
Text Solution
|
- The number of metamers of the compound with molecular formula C(5)H(12...
Text Solution
|