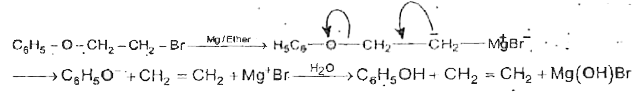Text Solution
Verified by Experts
Topper's Solved these Questions
ALKYL AND ARYL HALIDES
FIITJEE|Exercise EXERCISE -7|1 VideosALKYL AND ARYL HALIDES
FIITJEE|Exercise MATRIX-MATCH TYPE|1 VideosALKYL AND ARYL HALIDES
FIITJEE|Exercise EXERCISE -5|1 VideosALDEHYDES AND KETONES
FIITJEE|Exercise MATCHING LIST QUESTIONS|2 VideosATOMIC STRUCTURE
FIITJEE|Exercise NUMERICAL BASED QUESTIONS INDICATED BY DIGITAL INTEGER BY XXXXX.XX|2 Videos
Similar Questions
Explore conceptually related problems