Similar Questions
Explore conceptually related problems
Recommended Questions
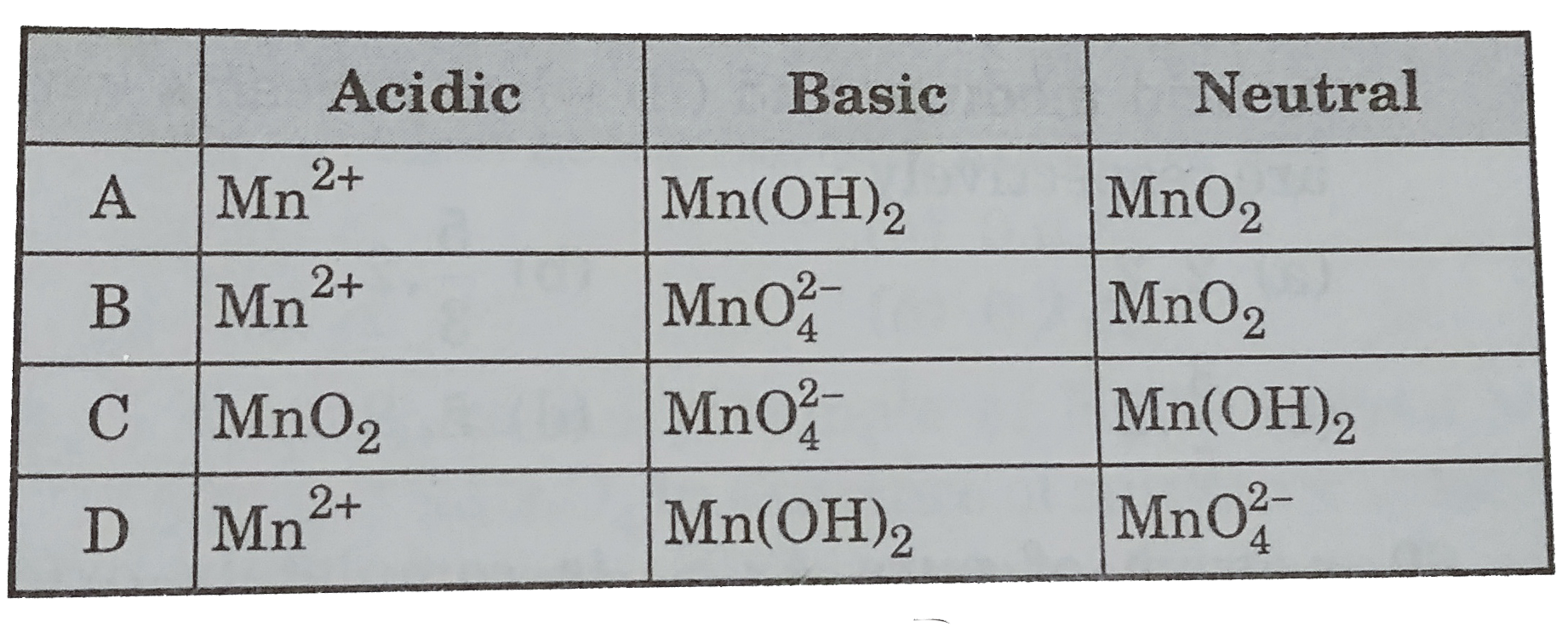
- When the permanganate ion, MnO(4)^(-),acts as an oxidizing agent it fo...
Text Solution
|
- In order to completely oxidize 0.1 mol of MnO(4)^(2-) to permanganate ...
Text Solution
|
- Assertion : In acid solution, permanganate is reduced to Mn^(2+) by an...
Text Solution
|
- Pottassium permanganate acts as an oxidant in neutral, alkaline as wel...
Text Solution
|
- Which species can act an oxidizing agent but not as a reducing agent ?
Text Solution
|
- When the permanganate ion, MnO(4)^(-) ,acts as an oxidizing agent it f...
Text Solution
|
- MnO(4)^(-) ions are reduced in acidic conditions to Mn^(2+) ions where...
Text Solution
|
- The ion(s) that act/s as oxidizing agent in solution is/are
Text Solution
|
- Write balanced equation for the oxidation of Ferrous ions to Ferric io...
Text Solution
|