A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
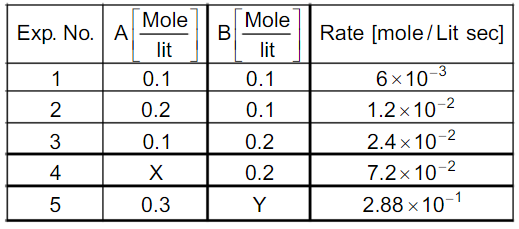
- For the reaction 2A +B rarr C Find X & Y.
Text Solution
|
- The rate equation for the reaction 2A+B rarr C is found to be: rate = ...
Text Solution
|
- For an elementary reaction, 2A + B rarr C + D the molecularity is
Text Solution
|
- 2A rarr B + C . It would be a zero-order reaction when
Text Solution
|
- For a reaction 2A +B rarr C+D, the active mass of B is kept constant b...
Text Solution
|
- 2A + B rarr C अभिक्रिया हेतु वेग नियम लिखिए।
Text Solution
|
- 2A rarr B+C . It would be a zero order reaction when.
Text Solution
|
- अभिक्रिया 2A + B rarr C D निम्न विधि से सम्पन्न होती है- 2A rarr A(2...
Text Solution
|
- For the reaction 2A +B rarr C Find X & Y.
Text Solution
|