Similar Questions
Explore conceptually related problems
Recommended Questions
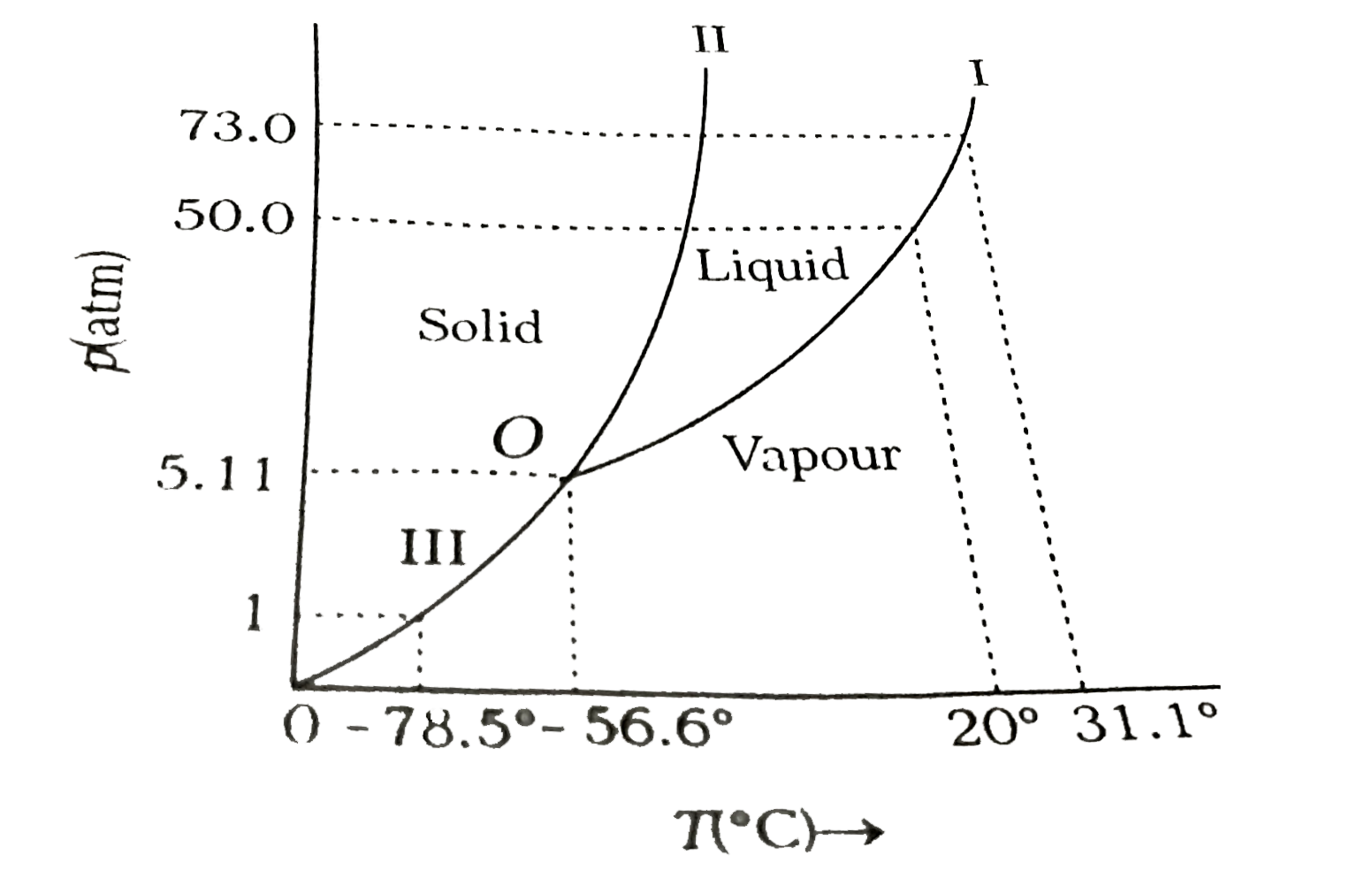
- Answer the following questions based on the p-T phase diagram of carbo...
Text Solution
|
- Answer the following question beased on tbhe P-T phase diagram of carb...
Text Solution
|
- Answer the following questions based on the p-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of carbo...
Text Solution
|