Similar Questions
Explore conceptually related problems
Recommended Questions
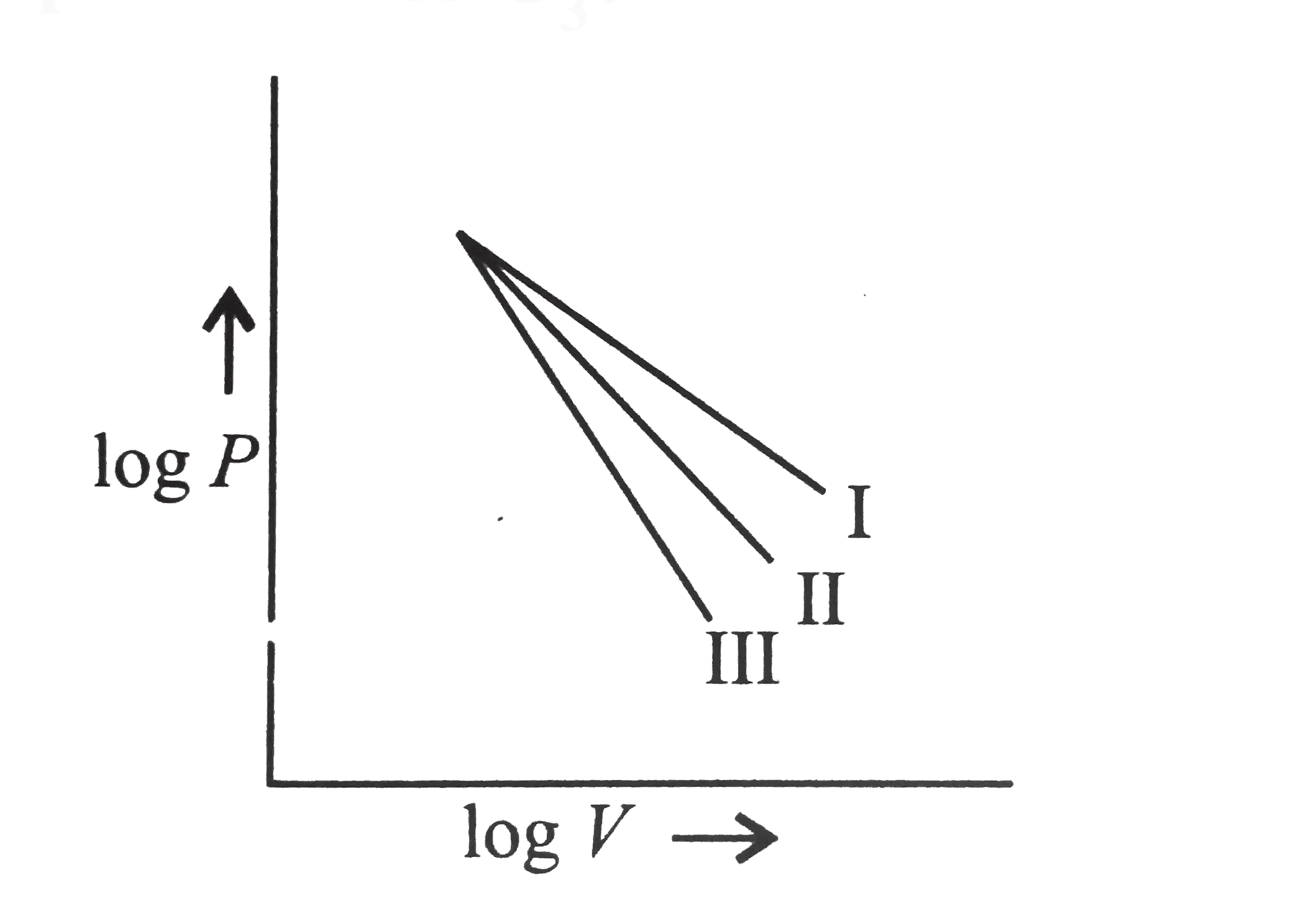
- The following curve represent adiabatic expansions of gases He,O(2),an...
Text Solution
|
- The following curve represent adiabatic expansions of gases He,O(2),an...
Text Solution
|
- He,N(2) , and O(3) are expanded adiabatically and their expansion curv...
Text Solution
|
- Bond order of O(2), O(2)^(-) and O(2)^(2-) is in order
Text Solution
|
- Which of the following order is correct for the bond dissociation ener...
Text Solution
|
- Which of the following produce Cr(2)O(3) along with O(2) ?
Text Solution
|
- O(2) dissocation curve is
Text Solution
|
- A mixture of equal mass of O(2) and O(3) gases are allowed to effuse t...
Text Solution
|
- O(2) dissociation curve is:
Text Solution
|