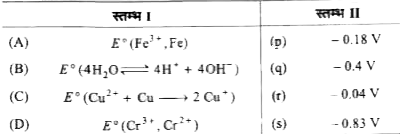जब ऑक्सीकरण-अपचयन (Redox) क्रिया में विभिन्न संख्या में इलेक्ट्रॉन भाग लेते हैं |
`Delta G_("कुल")^(@) = Delta G_(1)^(@) + Delta G_(2)^(@) - n_(3) FE_(3)^(@) = - n_(1)FE_(1)^(@) - n_(2) FE_(2)^(@)`
`therefore" "E_(3)^(@) = (n_(1)E_(1)^(@) + n_(2)E_(2)^(@))/(n_(3))`
(P) `E_(3)^(@) Fe^(3+) //Fe` अभिक्रिया `Fe^(3+) rarr Fe` प्राप्त होती है
`{:(,n" "E^(@)),(" "Fe^(3+) + e^(-) rarr Fe^(2+),bar(n_(1) = 1" "E_(1)^(@)=0.77V)),(" "Fe^(2+)+2e^(-) rarr Fe,n_(2)=2" "E_(2)^(@)=-0.44V),(because" "ulbar(Fe^(3+) + 3e^(-) rarr Fe),n_(3) = 3" "E_(3)^(@)=?):}`
`E_(3)^(@) = (n_(1)E_(1)^(@) + n_(2)E_(2)^(@))/(n_(3))`
`= (0.77 + 2(-0.44))/(3) = (-0.11)/(3) = - 0.04 V`
अत: `A rarr (r)`
अभिक्रिया `4H_(2)O harr 4H^(+) + 4OH^(-)` प्राप्त होती है
`{:(,ul(n" "E^(@))),(2H_(2)O rarr O_(2) + 4H^(+) + 4e^(-),n_(1) = 4" "-1.23 V),(2H_(2)O + O_(2) + 4e^(-) rarr 4OH^(-),n_(2) = 4" "+0.40 V),(" "ulbar(4H_(2)O rarr 4H^(+) + 4e^(-)),n_(3) = 4" "?):}`
`E_(3)^(@) = (n_(1)E_(1)^(@) + n_(2)E_(2)^(@))/(n_(3)) = E_(1)^(@) + E_(2)^(@)`
`=- 1.23 + 0.40 = - 0.83 V`
`B rarr (s)`
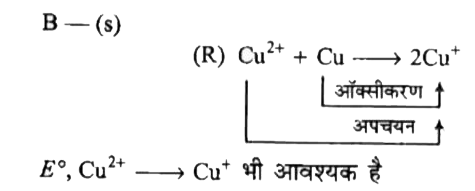
`E^(@), Cu^(2+) rarr Cu^(+)` भी आवश्यक है
`{:(,ul(n" "E^(@))),(Cu^(2+) + 2e^(-) rarr Cu,n_(1) = 2" "E_(1)^(@) = 0.34 V),(" "Cu rarr Cu^(+) + e^(-),n_(2) = 1" "-0.52 V),(ulbar(Cu^(2+) + e^(-) rarr Cu^(+))," "E_(3)^(@)" "?):}`
`E_(3)^(@) = (n_(1)E_(1)^(@) + n_(2) E_(2)^(@))/(n_(3)) = (2 xx 0.34 + 1 xx (-0.52))/(1) = 0.16 V`
अत:
`{:(,ul(n" "E^(@))),(" "Cu rarr Cu^(+) + e^(-),n_(1) = 1","" "-0.52 V),(Cu^(2+) + e^(-) rarr Cu^(+),n_(2) = 1" "0.10 V),(bar(Cu^(2+) + Cu rarr 2 Cu^(+)),):}`
`E^(@) = -0.52 + 0.16 = - 0,36 V`
`(C) rarr (p)`
(S) `Cr^(3+) rarr Cr^(2+)` प्राप्त होती है
`{:(,ul(n" "E^(@))),(Cr^(3+) + 3e^(-) rarr Cr,3" "-0.74 V),(" "Cr rarr Cr^(2+)+ 2e^(-),2" "+0.91 V),(" "ulbar(Cr^(3+) + e^(-) rarr Cr^(2+)),1" "?):}`
`E_(3)^(2) = (-0.74 xx 3 + 2 xx 0.91)/(1) = - 0.4 V" "S = (2)`
`P rarr (3), Q rarr (4), R rarr (1), S rarr (2)`