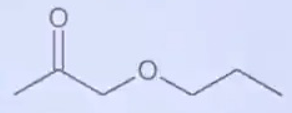
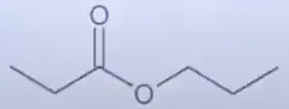
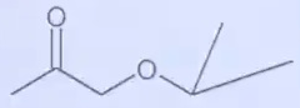
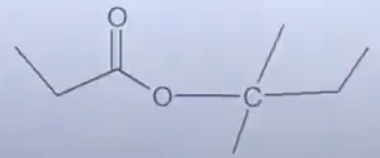
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A group C6H12O2 which react with H2SO4 and gives carboxylic acid B and...
Text Solution
|
- Primary alkyl halide C(4)H(9)Br (A) is reacted with alcoholic KOH to g...
Text Solution
|
- A nitraite on acid hydrolysis gives compound A, which reacts with thio...
Text Solution
|
- कार्बोओक्सिलिक अम्ल की ऐल्कोहॉल के साथ क्रिया से ................ बनता...
Text Solution
|
- Compound 'A' react with CH3-Cl gives B. B react with dil. H2SO4 gives ...
Text Solution
|
- A group C6H12O2 which react with H2SO4 and gives carboxylic acid B and...
Text Solution
|
- An organic compound (A) (molecular formula C6H(12)O2) was hydrolysed w...
Text Solution
|
- An organic compound (A) with molecularformula C2H5Cl reacts with KOH g...
Text Solution
|
- A is a king of acid. A reacts with HBr to give B and Bromine. A reacts...
Text Solution
|