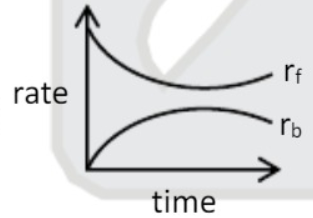
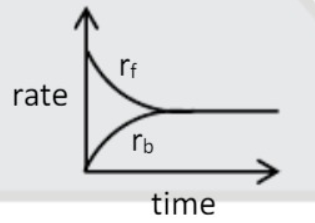
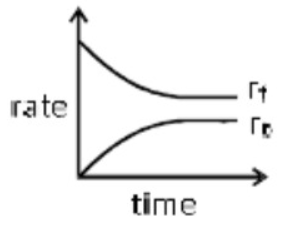
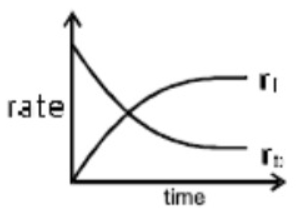
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- At equilibrium for a reaction A harr B. correct representation is {r...
Text Solution
|
- At equilibrium stage, the rate of forward reaction is ……….. To the rat...
Text Solution
|
- In the reaction A+B hArr C+D , the value of equilibrium constant is 10...
Text Solution
|
- In a chemical equilibrium, the rate constant for the backward reaction...
Text Solution
|
- In a chemical equilibrium, the rate constant for the backward reaction...
Text Solution
|
- In a chemical equilibrium , the rates of the forward and backwa...
Text Solution
|
- In the reaction A+B hArr C+D , the value of equilibrium constant is 10...
Text Solution
|
- At equilibrium for a reaction A harr B . correct representation is { r...
Text Solution
|
- At equilibrium rate of forward reaction becomes equal to rate of backw...
Text Solution
|