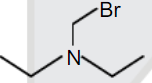
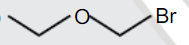
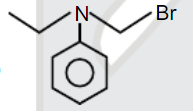
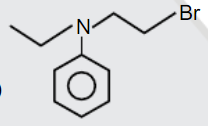
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one is most reaction towards AgNO3
Text Solution
|
- Which one is most reactive towards nucleophilic addition reaction?
Text Solution
|
- Which one of the following is most reactive towards nucleophilic subst...
Text Solution
|
- Which one is most reactive towards nucleophilic addition reaction?
Text Solution
|
- Which one of the following is most reactive towards nuvleophilic subst...
Text Solution
|
- Which one is most reactive towards Nucleophilic addition reaction
Text Solution
|
- Which one is most reactive towards nucleophilic addition reaction?
Text Solution
|
- The most reactive compound toward reaction with AgNO3 solution is :-
Text Solution
|
- Which one is most reactive towards nucleophilic addition reaction ?
Text Solution
|