Similar Questions
Explore conceptually related problems
Recommended Questions
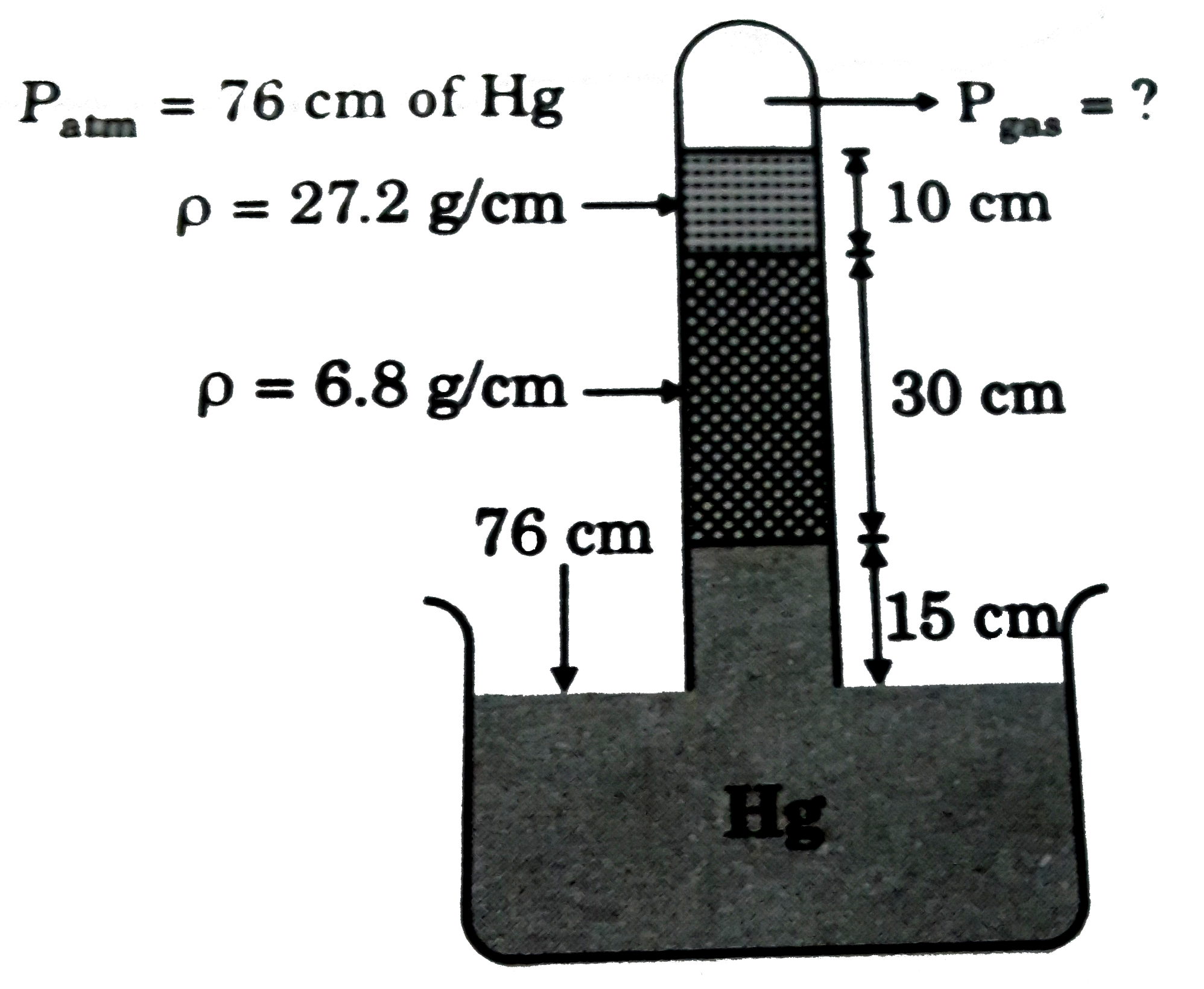
- In the following arrangement find the pressure of the confined gas in ...
Text Solution
|
- In the figure shown the pressure of the confined gas will be:
Text Solution
|
- A tube of length 50 cm is containing a gas in two secitons separated b...
Text Solution
|
- In the following arrangement find the pressure of the confined gas in ...
Text Solution
|
- A mass of gas exerts a pressure of 72 cm of Hg at 27^(@)C. It is heate...
Text Solution
|
- An 8 gram sample of a gas occupies 12.3 liters at a pressure of 40.0 c...
Text Solution
|
- The pressure of a gas filled in the bulb of a constant volume gas ther...
Text Solution
|
- When the bulb of standard gas thermometer is placed in melting ice, th...
Text Solution
|
- The pressures of a gas in constant volume gas thermometer are 100 cm a...
Text Solution
|