Similar Questions
Explore conceptually related problems
Recommended Questions
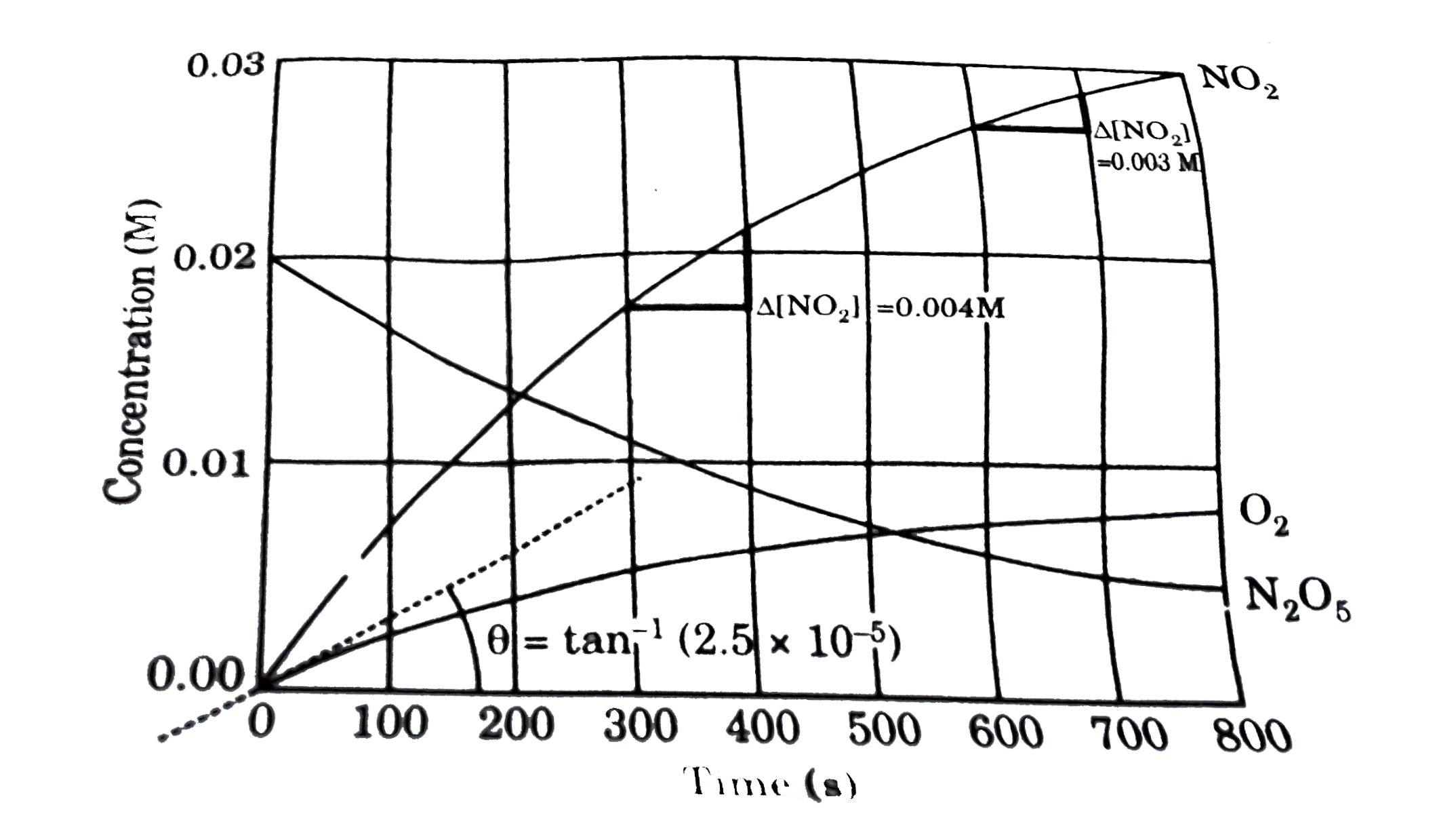
- Concentrations measured as a function of time when gaseous N(2)O(5) at...
Text Solution
|
- For the reactio 2N(2)O(5)to4NO(2)+O(2) The concentration of N(2)O(5)...
Text Solution
|
- The decomposition of N(2)O(5) takes place according to I order as: 2N(...
Text Solution
|
- The rate constant k , for the reaction N(2)O(5)(g) rarr 2NO(2) (g) + (...
Text Solution
|
- Gaseous N(2)O(5) decomposes according to the following equation: N(2...
Text Solution
|
- The rate cnstant k , for the reaction N(2)O(5)(g) rarr 2NO(2)(g) + (1)...
Text Solution
|
- Concentrations measured as a function of time when gaseous N(2)O(5) at...
Text Solution
|
- Concentrations measured as a function of time when gaseous N(2)O(5) at...
Text Solution
|
- Concentrations measured as a function of time when gaseous N(2)O(5) at...
Text Solution
|