Similar Questions
Explore conceptually related problems
Recommended Questions
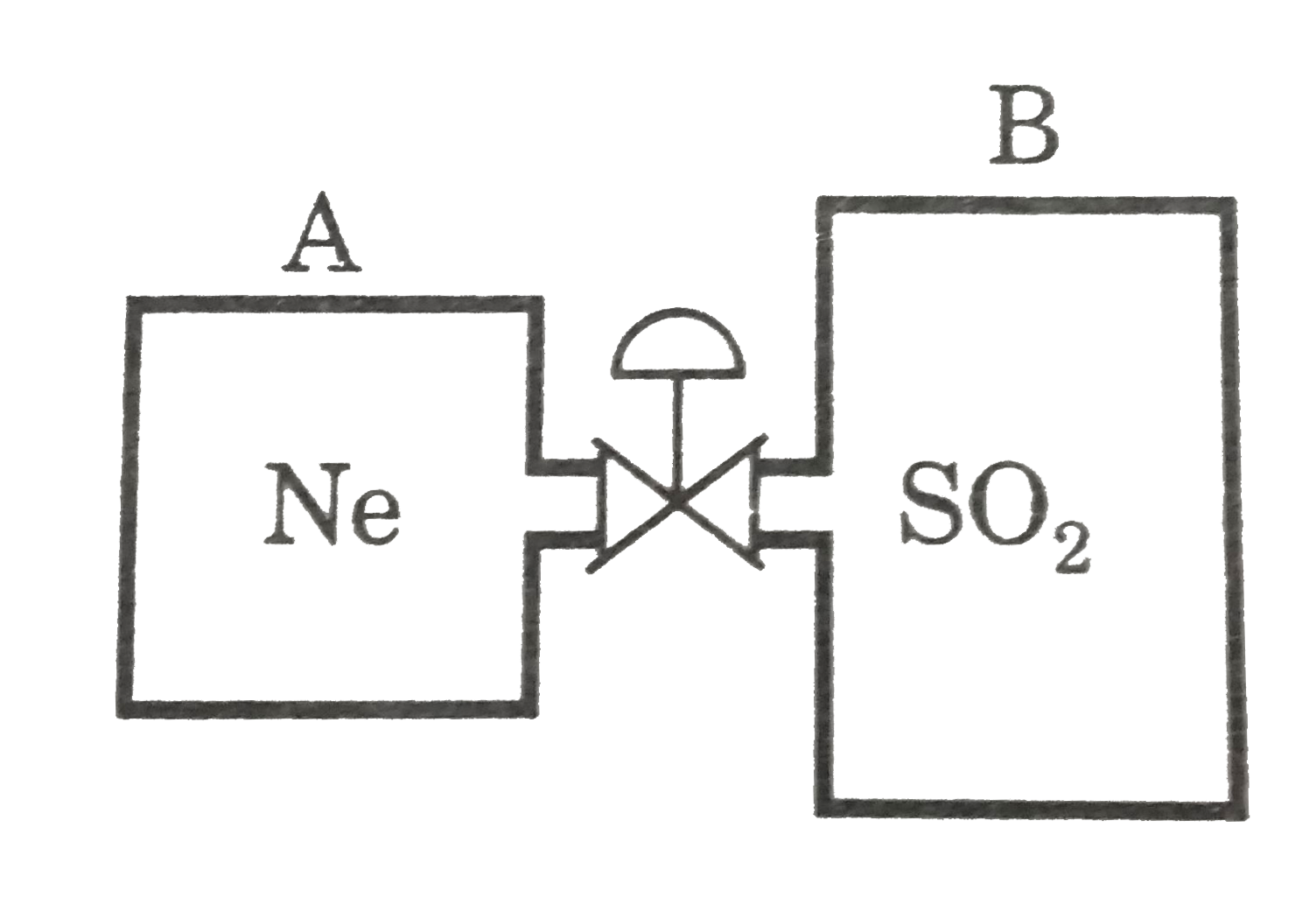
- Two rigid adiabatic vessel A and B which initially ,contain two gases...
Text Solution
|
- Two vessels A and B , thermally insulated, contain an ideal monoatomic...
Text Solution
|
- Two rigid adiabatic vessel A and B which initially ,contain two gases ...
Text Solution
|
- 2 moles of an ideal gas A is taken in an adiabatic container fitted wi...
Text Solution
|
- Two closed vessel A and B of equal volume of 8.21 L are connected by a...
Text Solution
|
- Three closed rigid vessels, A, B and C, which initially contain three ...
Text Solution
|
- If one mole of an ideal gas (C(p.m) = (5)/(2)R) is expanded isothermal...
Text Solution
|
- A closed vessel contains 0.1 mole of a monatomic ideal gas at 200k . ...
Text Solution
|
- Two closed vessel A and B of equal volume of 8.21 L are connected by a...
Text Solution
|