Similar Questions
Explore conceptually related problems
Recommended Questions
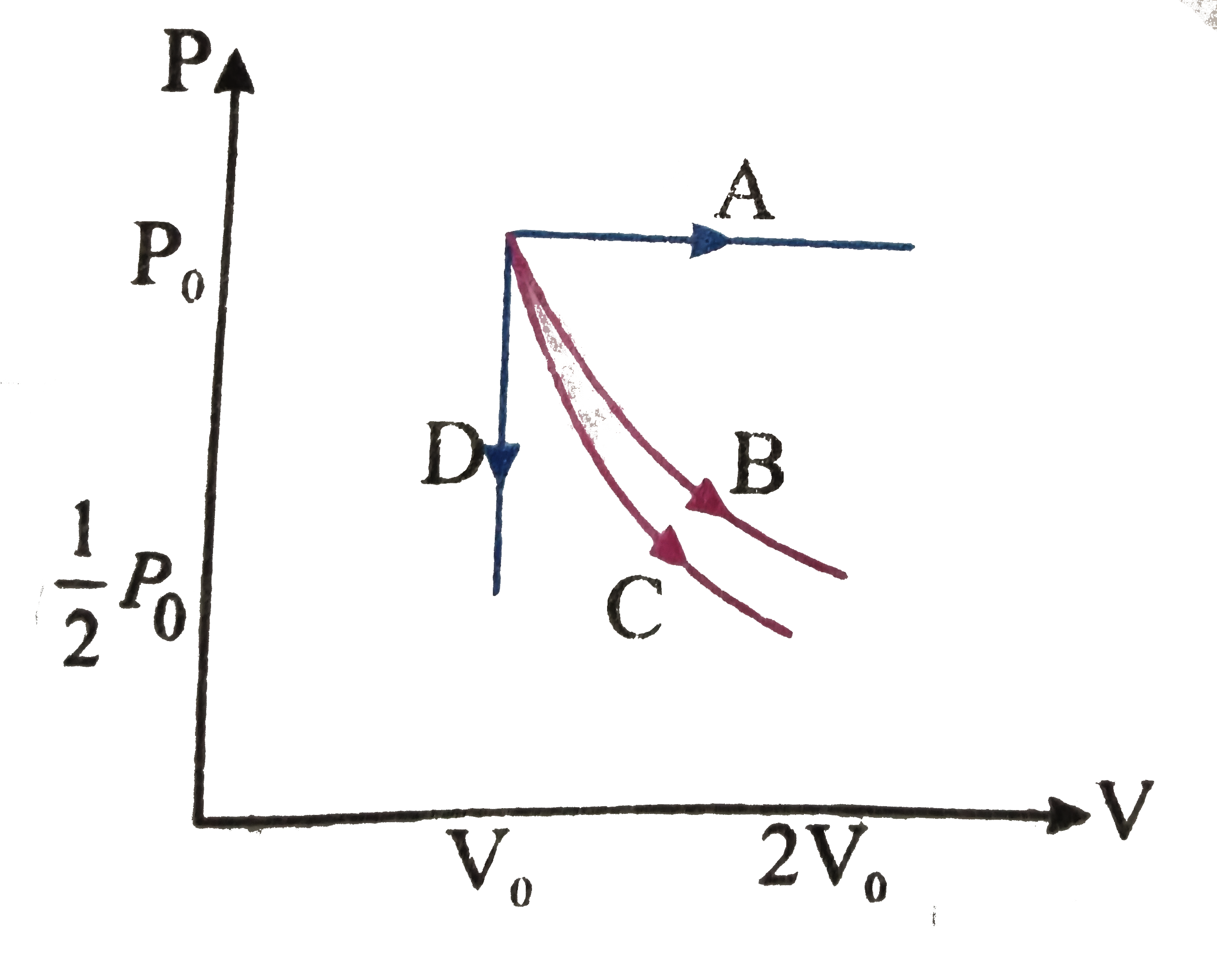
- The PV diagram shows four different possible paths of a reversible pro...
Text Solution
|
- How does the mean free path lamda and the number of collisions of each...
Text Solution
|
- PV = n RT holds good for A) Isobaric process B) Isochoric process C) I...
Text Solution
|
- The PV diagram shows four different possible paths of a reversible pro...
Text Solution
|
- 10 mol of an ideal gas (gamma= 1.5) expands adiabatically from (400K,...
Text Solution
|
- One mole of an ideal monoatomic gas is taken along the path ABCA as sh...
Text Solution
|
- One mole of ideal monoatomic gas is taken round the cyclic process as ...
Text Solution
|
- एकपरमाणुक आदर्श गैस के 1 मोल को चित्र में दिखाए गए चक्रीय प्रक्रम ABCA...
Text Solution
|
- An ideal gas undergoes four processes: isochoric, isobaric, isothermal...
Text Solution
|