A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
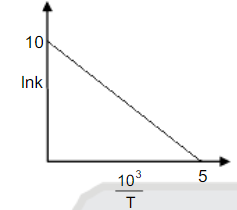
- Using following graph find activation energy (in KJ)
Text Solution
|
- An exothermic reaction XrarrY has an activation energy of 90kJ mol^(-1...
Text Solution
|
- An endothermic reaction A to B has an activation energy as K ...
Text Solution
|
- An endothermic reaction A to B has an activation energy as xx kJ mo1^(...
Text Solution
|
- An exothermic reaction A to B has an activation energy of X kJ mol^(-1...
Text Solution
|
- If a reaction A +B toC is exothermic to the extent of 30 kJ/mol and th...
Text Solution
|
- For a reaction XrarrY, heat of reaction is + a kJ, potential energy of...
Text Solution
|
- Using following graph find activation energy (in KJ)
Text Solution
|
- For A + B to C + D, DeltaH = -20 kJ "mole"^(-1) . The activation energ...
Text Solution
|