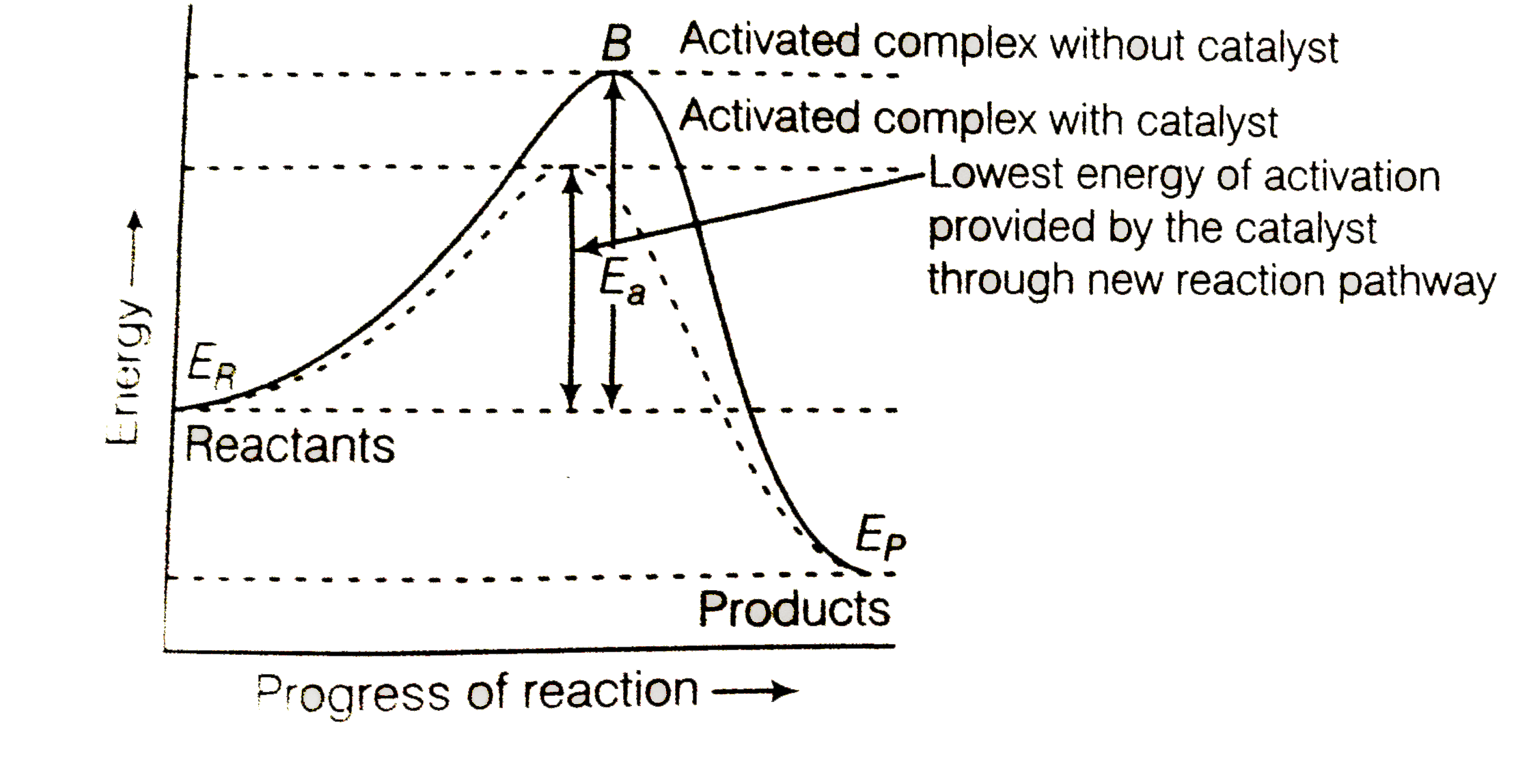
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-THERMODYNAMICS-QUESTIONS
- For a given reaction, DeltaH=35.5 KJ "mol"^(-1) and DeltaS=83.6 JK^(-1...
Text Solution
|
- A gas is allowed to expand in a well insulated container against a c...
Text Solution
|
- The addition of a catallystic during a chemical reaction alters which ...
Text Solution
|
- The correct thermodnamic conditions for the spontaneous reaction at al...
Text Solution
|
- Consider the following liquid-vapour equilibrium. "Liquid" hArr "Vap...
Text Solution
|
- For a sample of perfect gas when its pressure is changed isothermally ...
Text Solution
|
- Enthalpy of combustion of carbon to CO(2) is -393.5 kJ mol^(-1). Calcu...
Text Solution
|
- Which of the following statements is correct for a reversible process ...
Text Solution
|
- Which of the following statements of correct for the spontaneous adsop...
Text Solution
|
- For the reaction: X(2)O(4)(l)rarr2XO(2)(g) DeltaU=2.1 cal , DeltaS...
Text Solution
|
- For a given exothermic reaction , K(p) and k'(p) are the equilibrium c...
Text Solution
|
- A reaction having equal energies of activation for forward and reverse...
Text Solution
|
- In which of the following reactions,standard reaction entropy change(D...
Text Solution
|
- The enthalpy of fusion of water is1.435kcal//mol.The molar entropy cha...
Text Solution
|
- Standard enthalpy of vaporisationDeltaV(vap).H^(Theta) for water at 10...
Text Solution
|
- If the enthaply change for the transition of liquid water to steam is...
Text Solution
|
- Which of the following is the correct option for the free expansion of...
Text Solution
|
- Enthalpy change for the reaction 2H(2)(g)rarr4H(g) is -869.6kJ Th...
Text Solution
|
- The values of DeltaH and DeltaS for the reaction, C("graphite")+CO(2...
Text Solution
|
- From the following bond energies: H--H bond energy: 431.37KJmol^(-1) ...
Text Solution
|