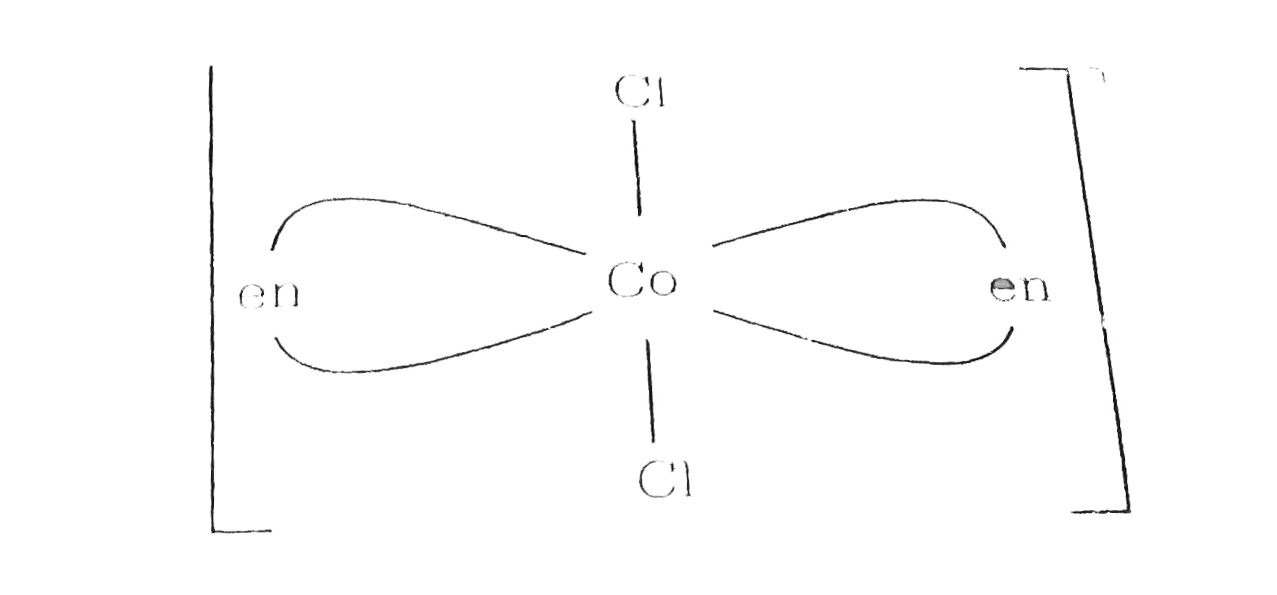
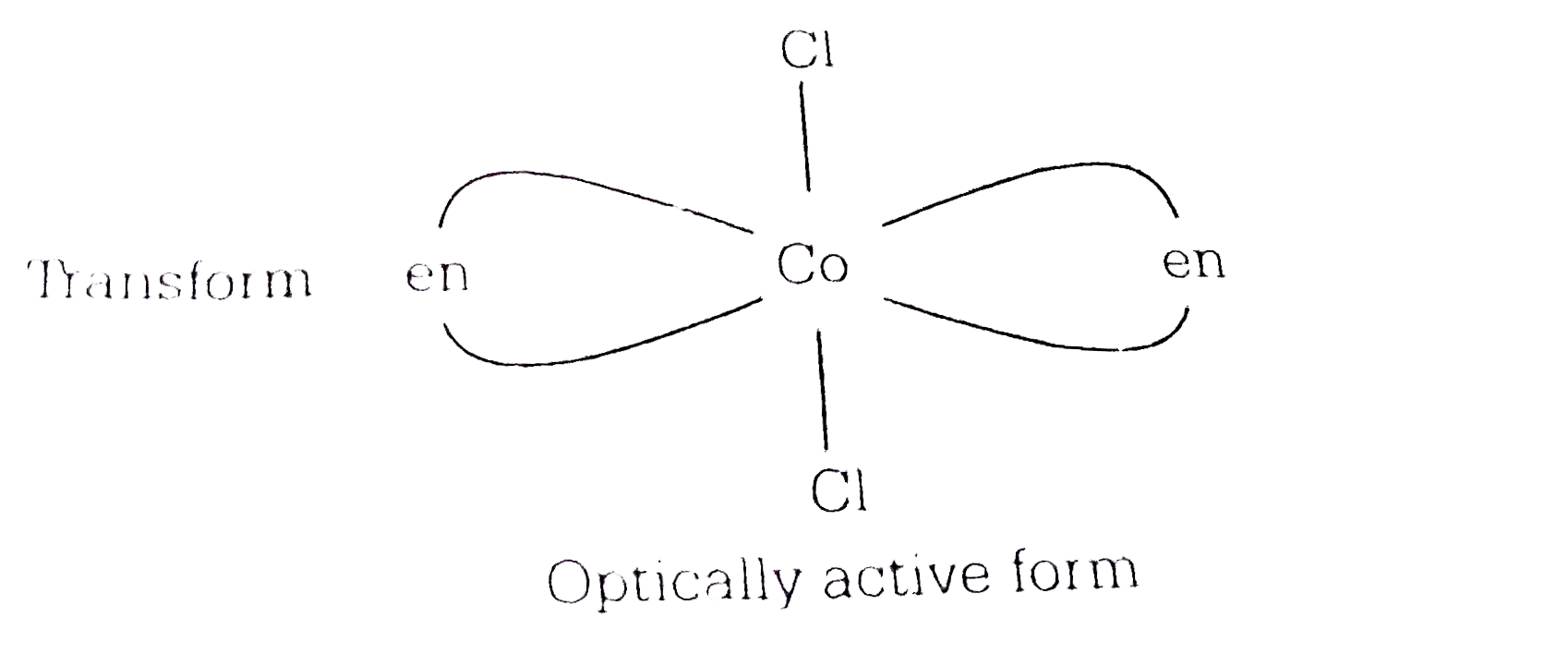
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-COORDINATION COMPOUNDS-EXERCISE
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- [Co(NH(3))(4)(NO(2))(2)]CI exhibits
Text Solution
|
- Which one of the following is expected to exhibit optical isomerism (e...
Text Solution
|
- Which of the following is an inner orbital complex as well as diamagne...
Text Solution
|
- Among |Ni(CO)(4)|, |Ni(CN)(4)|^(2-), |NiCl(4)|^(2-) species, the hybri...
Text Solution
|
- Considering H2O as a weak field ligand, the number of unpaired electro...
Text Solution
|
- CN^(-) is a strong field ligand. This is due to the fact that
Text Solution
|
- Which of the following does not have a metal carbon bond?
Text Solution
|
- Which of the following is considered to be an anticancer species?
Text Solution
|
- Which of the following coordination compounds would exhibit optical is...
Text Solution
|
- Among the following, which is not the pi-bonded organometallic compoun...
Text Solution
|
- The number of unpaired electrons in the complex ion [CoF(6)]^(3-) is
Text Solution
|
- According to IUPAC nomenclature sodium nitroprusside is named as
Text Solution
|
- Which of the following octahedral complex does not show geometrical is...
Text Solution
|
- The hypothetical complex chloro diaquatriammine cobalt (II) chloride c...
Text Solution
|
- Atomic numbers of Cr and Fe are respectively 24 and 26. Which of the f...
Text Solution
|
- Which of the following will give maximum number of isomer ?
Text Solution
|
- Which of the following organometallic compound is a sigma and pi bonde...
Text Solution
|
- Among |Ni(CO)(4)|, |Ni(CN)(4)|^(2-), |NiCl(4)|^(2-) species, the hybri...
Text Solution
|
- Which statement is incorrect?
Text Solution
|