Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN- CONCENTRATION TERMS-Basic Exercise
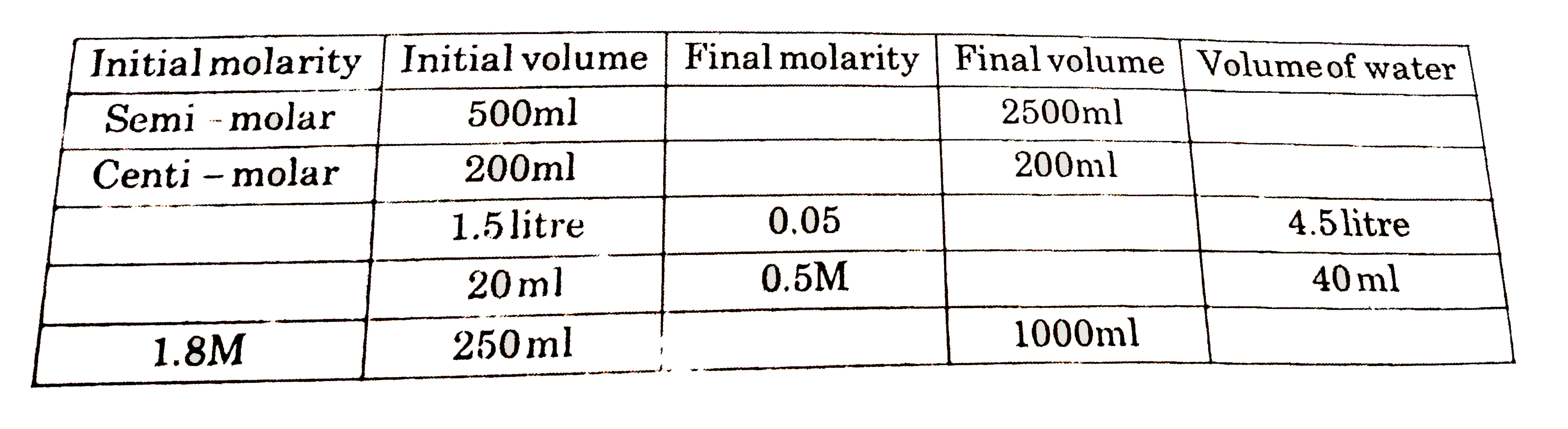
- Complete the following table (all are aqueous solution)
Text Solution
|
- (i) 2 gm H(2)SO(4) is dissolved in 38 gm water. Find (w/w)%
Text Solution
|
- 500 gm aq. NaOH solution contains 100 gm NaOH. Find (w/v)%
Text Solution
|
- A 200 ml aq. Solution of NaCl contains 20 gm of NaCl. Find (w/v)%
Text Solution
|
- A 2 litre solution of glucose contains 1 mole of glucose. Find (w/v)%
Text Solution
|
- 500 ml. aq. Solution of ethanol (C(2)H(5)OH) contains 20 ml of ethanol...
Text Solution
|
- 1 mole of glucose is dissolved in 4 moles of water to form a solution....
Text Solution
|
- 15 gm urea (NH(2)CONH(2)) is dissolved in 18 gm water to form an aq. S...
Text Solution
|
- 5.85 gm NaCl, 4 gm MgO is dissolved in 14.4 gm of water to form a solu...
Text Solution
|
- It was found that 20ml of water (d = 1 (gm)/(ml)) sample contains 0.5 ...
Text Solution
|
- The water (1250 kg) in an aquarium tank was with 5 mg of mercury. What...
Text Solution
|
- Use of dilution formula (M(1)V(1) = M(2) V(2))
Text Solution
|
- Calculate molarity of all the ions produces by following aq. Solution ...
Text Solution
|
- Find the resultant molarity obtained by mixing the following (i) 2 l...
Text Solution
|