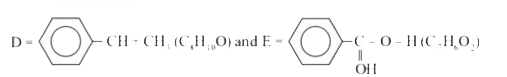Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
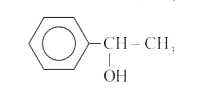
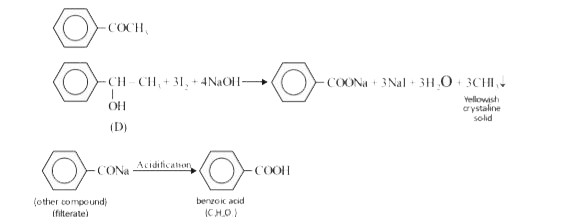
- A compound D(C(8)H(10)O) upon treatement with alkaline solution of iod...
Text Solution
|
- Compound (A) (C(8)H(8)O) on treatment with NH(2)OH.HCl gives (B) and (...
Text Solution
|
- A compound (D) (C(8)H(10)O) upon treatement with alkaline solution of ...
Text Solution
|
- A compound D(C(8)H(10)O) upon treatement with alkaline solution of iod...
Text Solution
|
- An aromatic compound A of the molecular formula C(8)H(10)O on reaction...
Text Solution
|
- An organic compound A (C(7)H(6)Cl(2)) on treatment with NaOH solution ...
Text Solution
|
- A compound A (C(8)H(10)O) upon treatment with alkaline solution of iod...
Text Solution
|
- An organic compound (A) of molecular formula C(5)H(8) when treated wit...
Text Solution
|
- अणुसूत्र C(3)H(6)O युक्त एक कार्बनिक यौगिक ( A) फेहलिंग विलयन के द्वार...
Text Solution
|