Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A) C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves ...
Text Solution
|
- Compound A (C(7)H(8)O) is insoluble in water , dilute HCL and aqueous ...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A) C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves ...
Text Solution
|
- A compound 'A' (C(7) H(8) O) is insoluble in water, dilute HCl and ...
Text Solution
|
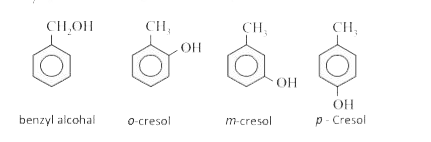
 Thus, the compound may be a m -cresol.
Thus, the compound may be a m -cresol.