A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
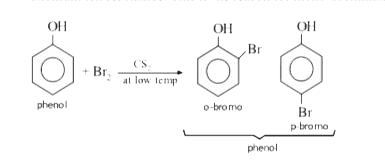
- Phenol reacts with bromine in carbon disulphide at low temperature to ...
Text Solution
|
- Phenol reacts with bromine in carbon disulphide at low temperature to ...
Text Solution
|
- Aniline reacts with bromine in CS(2) [carbon disulphide] at room tempe...
Text Solution
|
- When phenol reacts with bromine in CS(2) at a low temperature, the...
Text Solution
|
- Phenol reacts with bromine in chloroform at low temperature to give
Text Solution
|
- Phenol reacts with bromine in carbon disulphide at low temperature to ...
Text Solution
|
- Phenol reacts with bromine in chloroform at low temperature to give
Text Solution
|
- फिनोल निम्न ताप पर कार्बन डाईसल्फाइड में घुली ब्रोमीन से क्रिया करके न...
Text Solution
|
- Phenol reacts with bromine in CS2 at low temperature to give
Text Solution
|