A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
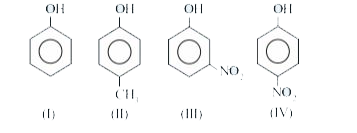
- The order of aciditic strength among the given phenols is :
Text Solution
|
- Among ethabol (I) Acetic acid (II) phenol (III) and Benzoic acid (IV)...
Text Solution
|
- Rank the following compounds in order of decreasing acid strength (mos...
Text Solution
|
- The correct order of acid strength of the following substituted phenol...
Text Solution
|
- Among ethanol (I), acetic acid (II), phenol (III) and benzoic acid (IV...
Text Solution
|
- The order of aciditic strength among the given phenols is :
Text Solution
|
- What is the increasing order of acidic strength among the following ? ...
Text Solution
|
- The correct order of acid strength of the following substituted phenol...
Text Solution
|
- Among ethanol (I) , Acetic acid (II), Phenol (III) and Benzoic acid (I...
Text Solution
|