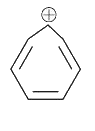
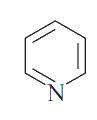
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY: SOME BASIC PRINCIPLES AND TECHNIQUES
CBSE COMPLEMENTARY MATERIAL|Exercise Fill in the blanks|10 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLES AND TECHNIQUES
CBSE COMPLEMENTARY MATERIAL|Exercise True and false type questions|10 VideosHYDROGEN
CBSE COMPLEMENTARY MATERIAL|Exercise Unit Test|11 VideosP-BLOCK ELEMENTS
CBSE COMPLEMENTARY MATERIAL|Exercise UNIT TEST|8 Videos
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-ORGANIC CHEMISTRY: SOME BASIC PRINCIPLES AND TECHNIQUES-Unit test
- which of the following species have six Pi constructed electrons?
Text Solution
|
- Write bond line formula for the following compound HOCH2CH2CH(CH3)CH(C...
Text Solution
|
- WRITE IUPAC name of the following compound: CH3-underset(NO2) underse...
Text Solution
|
- The cenral atom of compound ch2=c=ch2 is ........... Hybridized
Text Solution
|
- The difference in molecular weight of two consecutive members of a hom...
Text Solution
|
- What type of ismomerism is exhibvbited by the following pair of compou...
Text Solution
|
- Give one example each of nucleophile and electrophile
Text Solution
|
- Arrage thje following uin increasing order of staboltiy: (ch3)3c^-,ch3...
Text Solution
|
- Write two point of difference between inductive and electromeric effec...
Text Solution
|
- When do we use hot water funnel for filtration?
Text Solution
|
- How would you isolate a mixture of two organic compoundas which have d...
Text Solution
|
- An organic liqiuid decomposes below or at its boiling point. How will...
Text Solution
|
- Draw the resonating sturctures of 1. Phenol
Text Solution
|
- Draw the resonating sturctures of 2. benzaldehyde
Text Solution
|
- Arraange the following in the order of the order of property indicated...
Text Solution
|
- Arraange the following in the order of the order of property indicated...
Text Solution
|
- Arraange the following in the order of the order of property indicated...
Text Solution
|