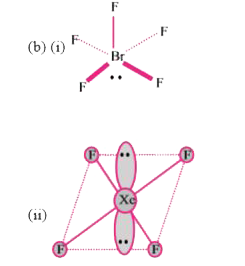
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-PRACTICE - PAPER-Section : D
- (i) Write the important structural and functional differences between ...
Text Solution
|
- (i) Calculate DeltaG^(@) and log K(c) for the following reaction at 29...
Text Solution
|
- (a) Write the product(s) in the following reactions : (i) (iii) C...
Text Solution
|
- (a) Account for the following: (i) Ozone is thermodynamically unstab...
Text Solution
|