Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
CBSE COMPLEMENTARY MATERIAL|Exercise SHORT ANSWER TYPE-II QUESTIONS|23 VideosCHEMICAL KINETICS
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS|12 VideosCHEMICAL KINETICS
CBSE COMPLEMENTARY MATERIAL|Exercise VERY SHORT ANSWER TYPE QUESTIONS|24 VideosBIOMOLECULES
CBSE COMPLEMENTARY MATERIAL|Exercise SHORT ANSWER-II TYPE QUESTION|13 VideosCHEMISTRY IN EVERYDAY LIFE
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS|2 Videos
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-CHEMICAL KINETICS-SHORT ANSWER-I TYPE QUESTIONS
- The decomposition of hydrocarbon follows the equation k=(4.5xx10^(11)...
Text Solution
|
- A reaction is of second order with respect to a reactant. How is the r...
Text Solution
|
- For a first order reaction, time taken for half of the reaction to com...
Text Solution
|
- What is the order of the reaction ?
Text Solution
|
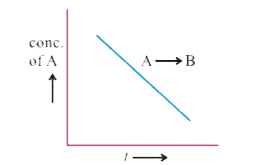
- What is the slope of the curve ?
Text Solution
|
- Derive an expression to calculate time required for completion of zero...
Text Solution
|
- For the reactionN2 (g) + 3H2 (g) to 2NH3 (g) How is the rate of for...
Text Solution
|
- The rate of a gaseous reaction is halved when the volume of the vessel...
Text Solution
|
- A reaction which is first order with respect to A has rate constant 6 ...
Text Solution
|
- The conversion of the molecules X to Y follows second order kinetics. ...
Text Solution
|
- A first order reaction has a rate constant of 1.15xx10^(-3) s^(-1). Ho...
Text Solution
|
- 4NH3 + 5O2 to 4NO + 6H2O. If rate of formation of NO is 6 xx 10^(−4) "...
Text Solution
|
- Consider a certain reaction A rarr Products with k=2.0xx10^(-2)s^(-1)....
Text Solution
|
- Explain with an example, what is a pseudo first order raction ? The gr...
Text Solution
|
- Differentiate between :Average rate and instantaneous rate of a chemic...
Text Solution
|
- Differentiate between :Molecularity and order of reaction.
Text Solution
|
- Show that in case of a first order reaction, the time required for 99....
Text Solution
|
- For the reaction NO2 + CO to CO2 + NO, the experimentally determined r...
Text Solution
|
- The half life period of a first order reaction is 60 min. What percent...
Text Solution
|
- Time for half change for a first order reaction is 25 min. What time w...
Text Solution
|