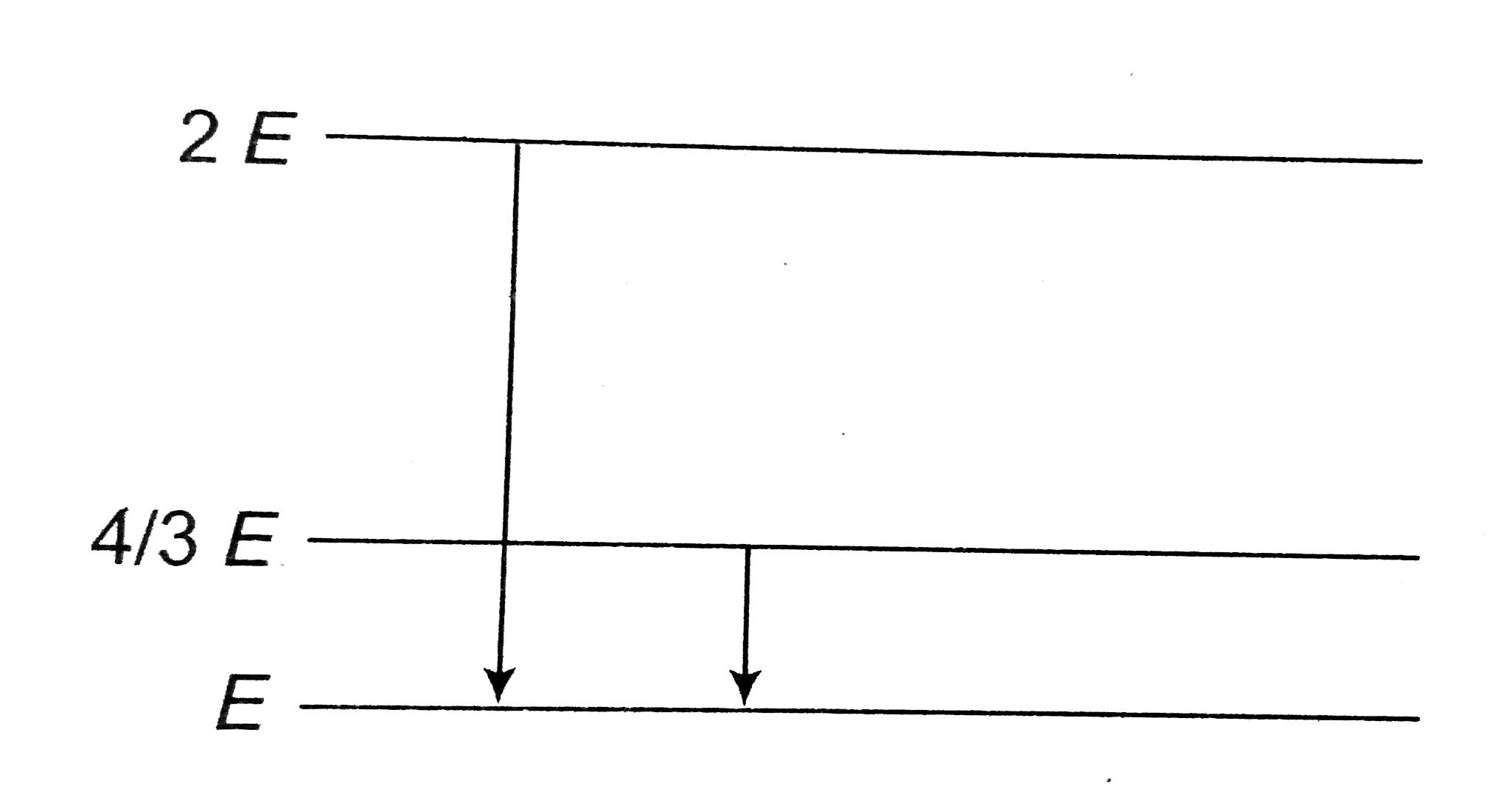
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMS -EXERCISE -4
- A photon of energy 15eV collides with H-atom. Due to this collision, H...
Text Solution
|
- Monochromatic radiation of wavelength lambda is incident on a hydrogen...
Text Solution
|
- The follwing diagram indicates the energy levels of a certain atom whe...
Text Solution
|
- When the electron in a hydrogen atom jumps from the second orbit to th...
Text Solution
|
- The ratio of the largest to shortest wavelength in Balmer series of hy...
Text Solution
|
- The electron in hydrogen atom makes a transition n(1)ton(2) where n1 a...
Text Solution
|
- Any radiation in the ultraviolet region of hydrogen spectrum is able t...
Text Solution
|
- A hydrogen atom emits a photon corresponding to an electron transition...
Text Solution
|
- The wave number of energy emitted when electron jumps from fourth orbi...
Text Solution
|
- In a Bohr atom the electron is replaced by a particle of mass 150 time...
Text Solution
|
- If the wavelength of the first member of Balmer series of hydrogen spe...
Text Solution
|
- A hydrogen like atom (atomic number Z) is in a higher excited state of...
Text Solution
|
- Photon from n=2 to n=1 in hydrogen atom is made to fall on a metal su...
Text Solution
|
- Let nu(1) be the frequency of the series limit of the lyman series n...
Text Solution
|
- (a) (i) Find the wavelength of the radiation required to excite thye e...
Text Solution
|
- Find the wavelength in a hydrogen spectrum between the range 500nm to ...
Text Solution
|
- The largest wavelength in the ultraviolet region of the hydrogen spect...
Text Solution
|
- The electric potential between a proton and as electron is given by V=...
Text Solution
|
- If elements of quantum number greater than n were not allowed , the nu...
Text Solution
|
- Magnetic field at the center (at nucleus) of the hydrogen like atom ("...
Text Solution
|