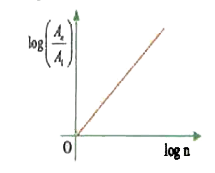
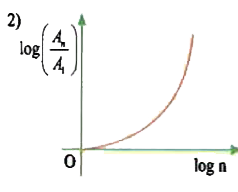
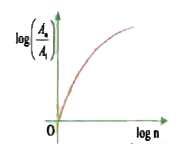
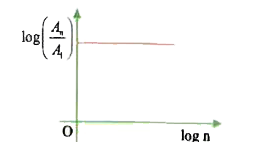
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-ATOMS -EXERCISE -4
- If the wavelength of the first member of Balmer series of hydrogen spe...
Text Solution
|
- A hydrogen like atom (atomic number Z) is in a higher excited state of...
Text Solution
|
- Photon from n=2 to n=1 in hydrogen atom is made to fall on a metal su...
Text Solution
|
- Let nu(1) be the frequency of the series limit of the lyman series n...
Text Solution
|
- (a) (i) Find the wavelength of the radiation required to excite thye e...
Text Solution
|
- Find the wavelength in a hydrogen spectrum between the range 500nm to ...
Text Solution
|
- The largest wavelength in the ultraviolet region of the hydrogen spect...
Text Solution
|
- The electric potential between a proton and as electron is given by V=...
Text Solution
|
- If elements of quantum number greater than n were not allowed , the nu...
Text Solution
|
- Magnetic field at the center (at nucleus) of the hydrogen like atom ("...
Text Solution
|
- The recoil speed of a hydrogen atom after it emits a photon in going f...
Text Solution
|
- The binding energy of an electron in the ground state of He atom is E(...
Text Solution
|
- In hydrogen atom, the radius of n^(th) Bohr orbit is r(n). The graph B...
Text Solution
|
- In hydrogen atom, the area enclosed by n^(th) orbit is A(n). The graph...
Text Solution
|
- An electron in the ground state of hydrogen atom is revolving in antic...
Text Solution
|
- if we assume only gravitational attraction between proton and electro...
Text Solution
|
- Taking the Bohr radius a(0) = 53 pm, the radius of Li^(++) ion in its ...
Text Solution
|
- The simple Bohr model cannot be directly ap-plied to calculate the ene...
Text Solution
|
- Two H atoms in the ground state collide in elastically. The maximum am...
Text Solution
|
- A set of atom in an excited state decays
Text Solution
|