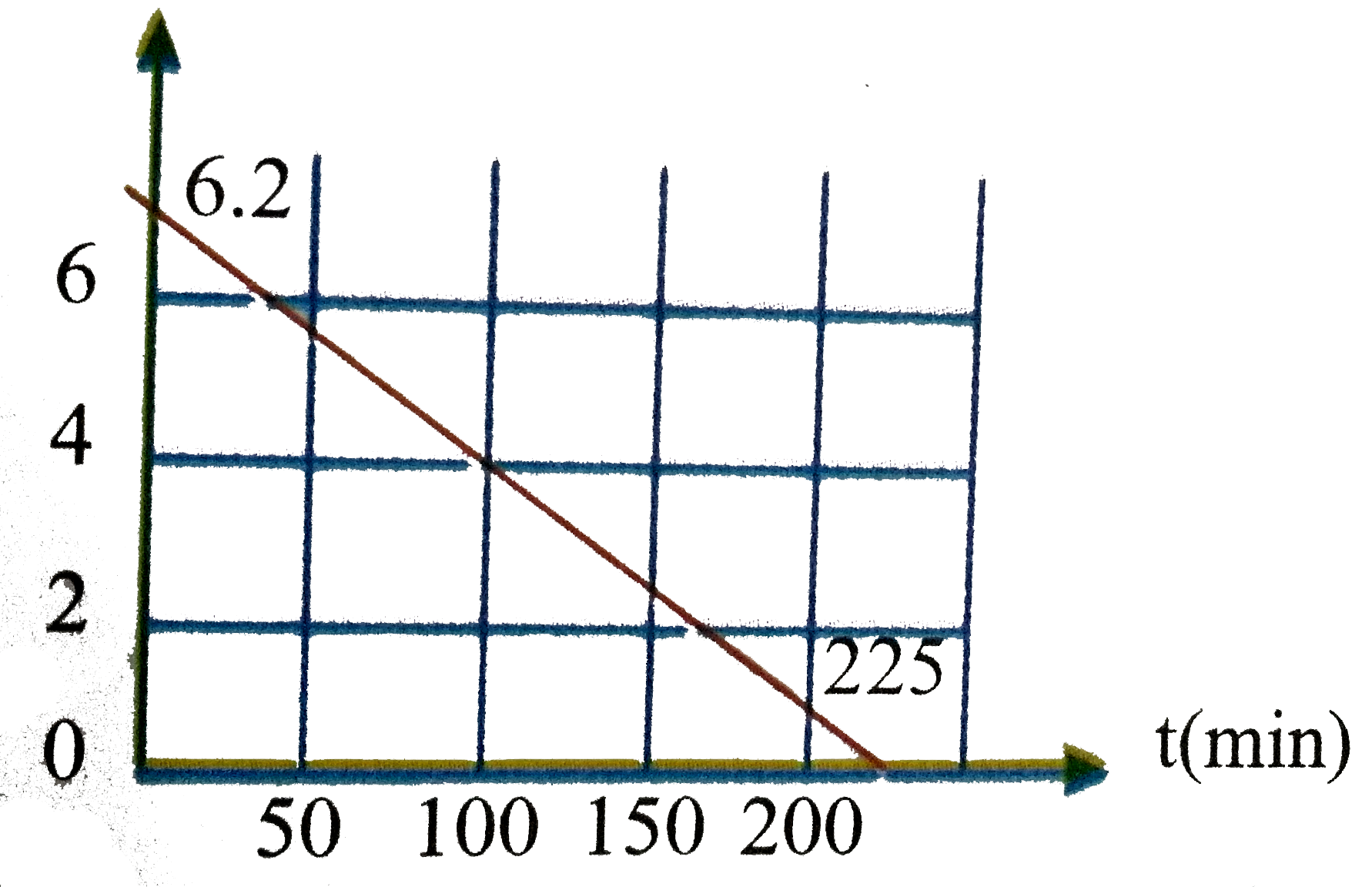
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-NUCLEI -EXERCISE-4
- If m g of a radioactive species (molar mass M) has decay constant lamb...
Text Solution
|
- In moon rock sample the ratio of the number of stable argon-40 atoms p...
Text Solution
|
- The half-life of a radioactive sample is T. If the activities of the s...
Text Solution
|
- Consider a hypothetical annihilation of a stationary electron with a s...
Text Solution
|
- A radioactive nucleus can decay by two different processes. The half l...
Text Solution
|
- A proton with kinetic energy K, strikes another proton at rest. If the...
Text Solution
|
- The fraction quantity of a radiactive sample will decay during half of...
Text Solution
|
- A small quantity of a solution containing Na^(24) radio-nuclide of ha...
Text Solution
|
- A nucleus with mass number 220 initially at rest emits an alpha-partic...
Text Solution
|
- Some amount of a radioactive substance (half-life= 10 days) is spread ...
Text Solution
|
- In the options given below, let E denote the rest mass energy of a nuc...
Text Solution
|
- Four different radioactive elements are kept in separated containers. ...
Text Solution
|
- Binding energy per nucleons vs mass curve for nucleus is shown in the ...
Text Solution
|
- When (3)Li^(7) nuclei are bombarded by protons , and the resultant nuc...
Text Solution
|
- A sample of uranium is a mixture of three isotopes .(92)U^(234), .(92)...
Text Solution
|
- The table that follows shows some measurements of the decay rate of a ...
Text Solution
|
- The fraction f of radioactive material that has decayed in time t, var...
Text Solution
|
- The rate of decay (R ) of nuclei in a radioactive sample is plotted ag...
Text Solution
|
- What is the probability of a radioactive nucleus to survive one mean l...
Text Solution
|
- A radioactive isotope is being produced at a constant rate dN//dt=R in...
Text Solution
|