Text Solution
Verified by Experts
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Solved Example|74 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Illustrations of Objective Questions|58 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Self Assessment (Integer type).
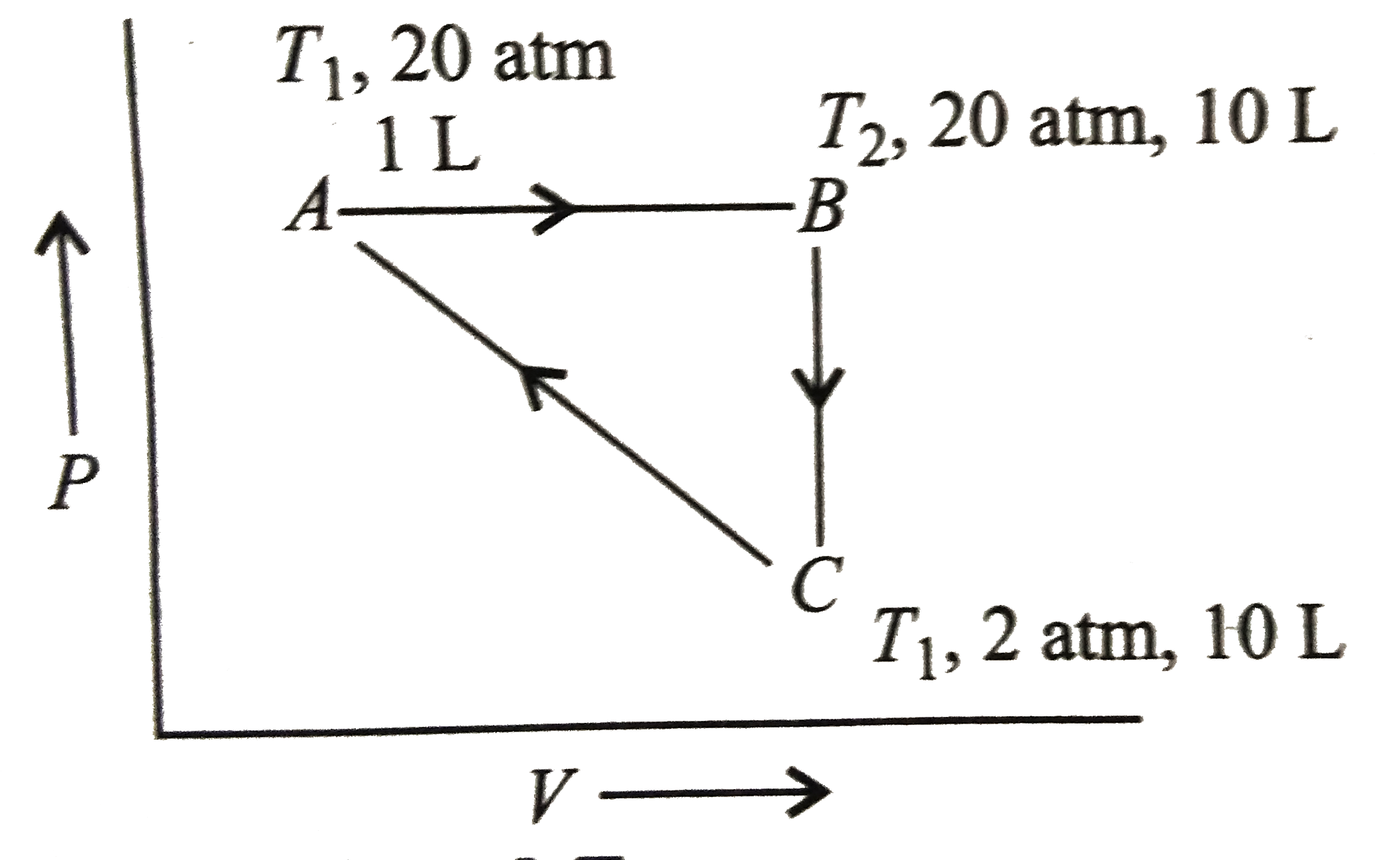
- 1mol of a mono-atomic gas is subjected to following cyclic process: ...
Text Solution
|
- heat of neutralisation of HCl against NaOH is 13.7 kcal eq^(-1) what w...
Text Solution
|
- Free energy change for the process A(s)hArrA(l) will be:
Text Solution
|
- A gas X(n) has the value of gamma equal to 1.40 : what will be the val...
Text Solution
|