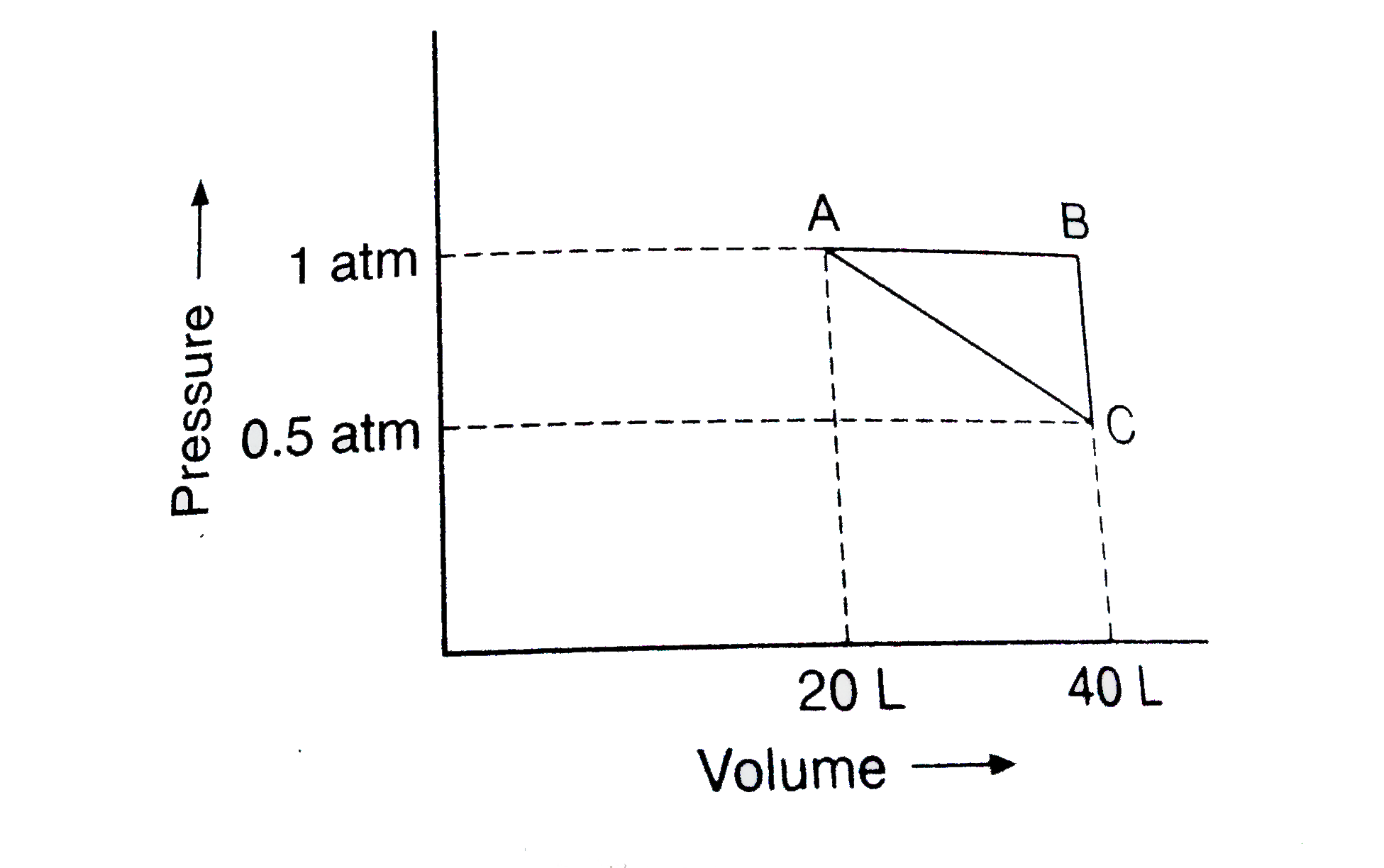Text Solution
Verified by Experts
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Practice problems|81 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level-A|177 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Illustrations of Objective Questions|58 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Miscellaneous Numerical Examples
- The standared heat of formation listed for gaseous NH(3) is -11.0kcal ...
Text Solution
|
- The heat of combusion of glycogen is about 476 kJ mol^(-1) of carbon. ...
Text Solution
|
- At 25^(@)C, the following heat of formations are given: {:("Compound...
Text Solution
|
- The 'heat of total cracking' of hydrocarbons DeltaH(TC) is defined as ...
Text Solution
|
- A constant pressure calorimeter consists of an insulated beaker of mas...
Text Solution
|
- C(2)H(4) +CI(2) rarr C(2)H(4)CI(2) DeltaH =- 270.6 kJ mol^(-1)K^(-1)...
Text Solution
|
- Find bond enthalpy of S-S bond from the following data: C(2)H(5)-S-C...
Text Solution
|
- A natural gas may be assumed to be a mixture of methane and ethane onl...
Text Solution
|
- From the data at 25^(@)C: Fe(2)O(3)(s) +3C(("graphite")) rarr 2Fe(s)...
Text Solution
|
- Calculate DeltaH at 85^(@)C for the reaction: Fe(2)O(3)(s) +3H(2)(g)...
Text Solution
|
- The standard heats of formation at 298K for C Cl(4)(g),H(2)O(g),CO(2)(...
Text Solution
|
- Calculateq, W, DeltaU and DeltaH for the isothermal reversible expansi...
Text Solution
|
- A sample of argon gas at 1atm pressure and 27^(@)C expands reversibly ...
Text Solution
|
- Show that the reaction CO(g) +(1//2)O(2)(g) rarr CO(2)(g) at 300K ...
Text Solution
|
- Diborane is a potential rocket fuel which undergoes combustion accordi...
Text Solution
|
- An insulated vessel contains 1mole of a liquid, molar volume 100mL at ...
Text Solution
|
- In the reaction equilibrium N(2)O(4) hArr 2NO(2)(g) When 5 mol of ...
Text Solution
|
- When 1pentyne (A) is treated with 4N alcoholic KOH at 175^(@)C, it is ...
Text Solution
|
- Two moles of a perfect gas undergo the following processes: a. A rev...
Text Solution
|
- The surface of copper gets tarnished by the formation of copper oxide....
Text Solution
|