Text Solution
Verified by Experts
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level-A|177 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Set 2|18 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Miscellaneous Numerical Examples|22 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Practice problems
- Write 'yes' if heat, work or matter are able to cross the boundary of ...
Text Solution
|
- Which of the following are state functions? (i) Q (ii) w (iii). ...
Text Solution
|
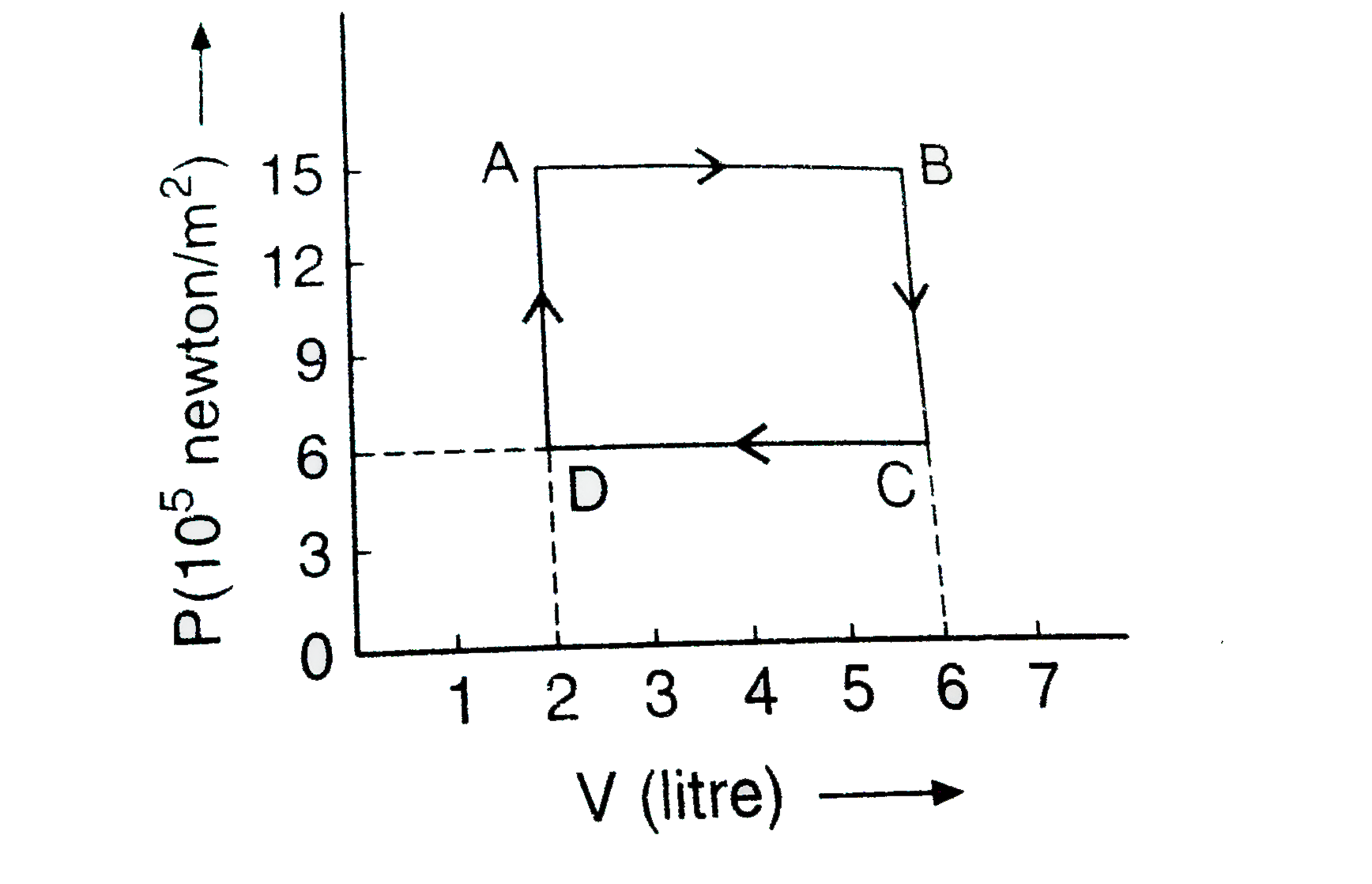
- In the adjoining diagram, the p-V graph of an ideal gas is shown. Find...
Text Solution
|
- A sample of a gas contracts 200cm^(3) by an average of .5 atmosphere w...
Text Solution
|
- Calculate the pressure-volume work by the system when the gas expands ...
Text Solution
|
- A sample of a gas in a cyclinder contracts by 7.5 litre at a constant ...
Text Solution
|
- A sample of a gas expands from 200cm^(3) to 500cm^(3) against an avera...
Text Solution
|
- Calculate the work done when 65.38 g of zinc dissolves in hydrochloric...
Text Solution
|
- 6 moles of an ideal gas expand isothermally and reversible from a volu...
Text Solution
|
- 1 mole of a ideal gas at 25^(@)C is allowed to expand reversibly and i...
Text Solution
|
- 1 mole of a ideal gas at 25^(@)C is allowed to expand reversibly and i...
Text Solution
|
- How much energy is absorbed by 10 moles of an ideal gas if it expands ...
Text Solution
|
- A given mass of a gas at 0^(@)C is compressed reversible and adiabatic...
Text Solution
|
- 3 moles of hydrogen are compressed isothermally and reversible from 60...
Text Solution
|
- To what pressure must a certain ideal gas (gamma=1.4) at 373 K and 1 a...
Text Solution
|
- 1 mole of an ideal gas (C(V)=12.55JK^(-1)" "mol^(-1)) at 300K is compr...
Text Solution
|
- Calculate the internal energy change for the process in which 1.0 kcal...
Text Solution
|
- Calculate DeltaU and DeltaH when 10dm^(3) of helium at NTP is heated i...
Text Solution
|
- For the conversion of 1 mole of SO(2)(g) into SO(3)(g) the enthalpy of...
Text Solution
|
- The heat liberated on complete combustion of 7.8 g benzene is 327 kJ.T...
Text Solution
|