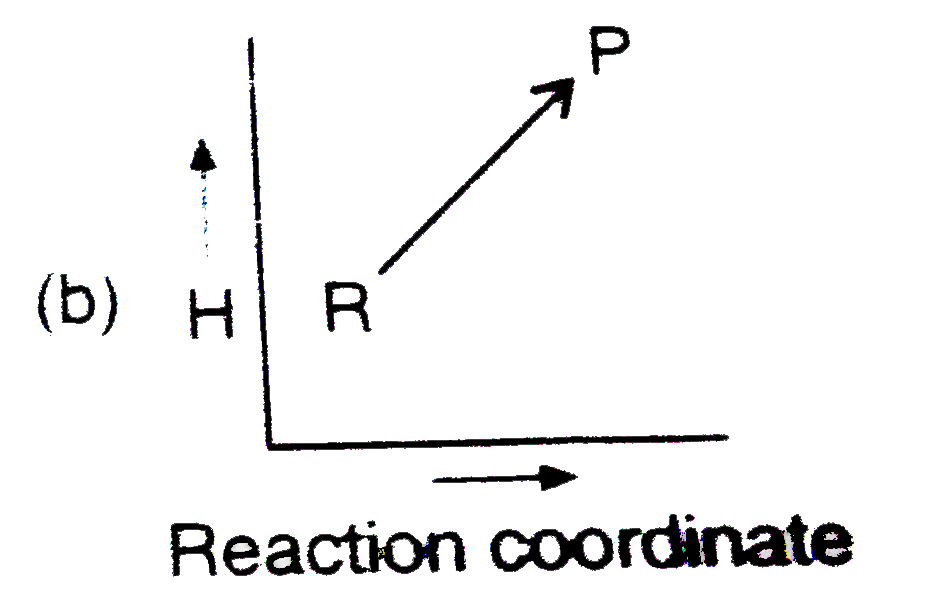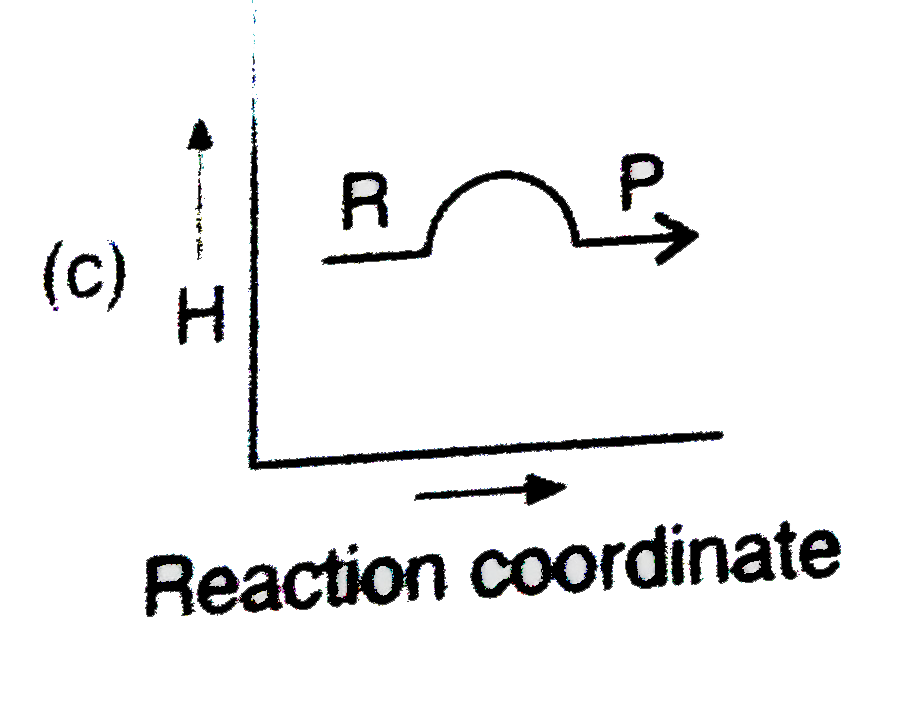A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Set 2|18 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level B|56 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Practice problems|81 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Objective Question Level-A
- Latent heat of vaporisation of a liquid at 500K and 1 atm pressure is ...
Text Solution
|
- The enthalpy change of a reaction does not depend on
Text Solution
|
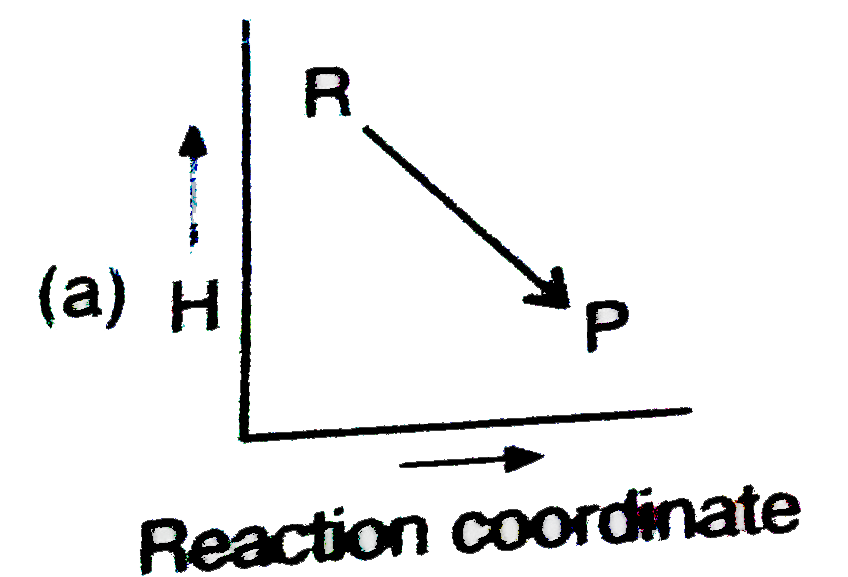
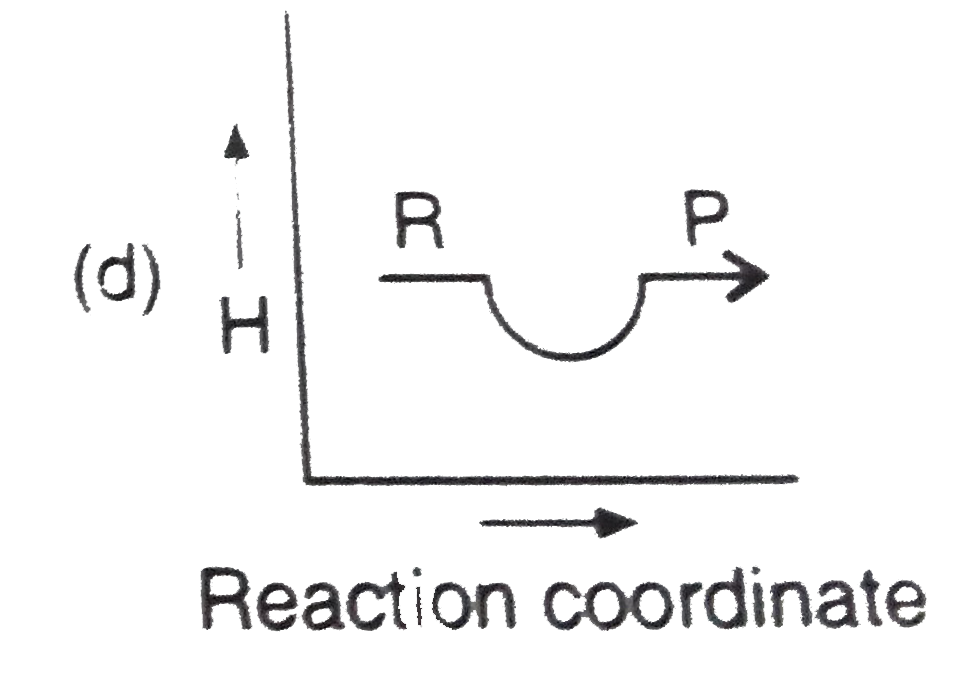
- Which plot represents for an exothermic reaction ?
Text Solution
|
- Given : S((s))+(3)/(2)O(2(g))rarrSO(3(g)+2X Kcal SO(2(s))+(1)/(2)O(2...
Text Solution
|
- NH(3)(g)+3Cl(2)toNCl(3)(g)+3HCl(g),DeltaH(1) N(2)(g)+3H(2)(g)to2NH(3...
Text Solution
|
- The word 'standard' in standard molar enthalpy change implies
Text Solution
|
- The heat of formation (DeltaH(f)^(@)) of H(2)O(l) is equal to :
Text Solution
|
- The value of DeltaH and DeltaS for a reaction are respectively 30 kJ m...
Text Solution
|
- The value of DeltaH^(@) for the reaction Cu^(+)(g)+I^(-)(g)toCuI(g) is...
Text Solution
|
- If the enthalpy change for the reaction, CH(4)(g)+Cl(2)(g)toCH(3)Cl(...
Text Solution
|
- The standard heat of formation of sodium ions in aqueous solution from...
Text Solution
|
- AB,A(2) and B(2) are diatomic molecules. If the bond enthalpies of A(2...
Text Solution
|
- The lattice energy of solid NaCI is 180 kcal mol^(-1). The dissolution...
Text Solution
|
- Which one of the following statement is false?
Text Solution
|
- DeltaG^(ɵ) for the reaction X+YhArrC is -4.606 kcal at 1000 K. The equ...
Text Solution
|
- The enthalpy of dissolution of BaCl(2)(s) and BaCl(2).2H(2)O are -20.6...
Text Solution
|
- For the reaction A(g)+2B(g)rarr2C(g)+3D(g), the value of DeltaH at...
Text Solution
|
- In thermodynamics, a process is called reversible when
Text Solution
|
- The heat liberated when 1.89 g of benzoic acid is burnt in a bomb calo...
Text Solution
|
- One mole of a non-ideal gas undergoes a change of state (2.0atm,3.0L,9...
Text Solution
|