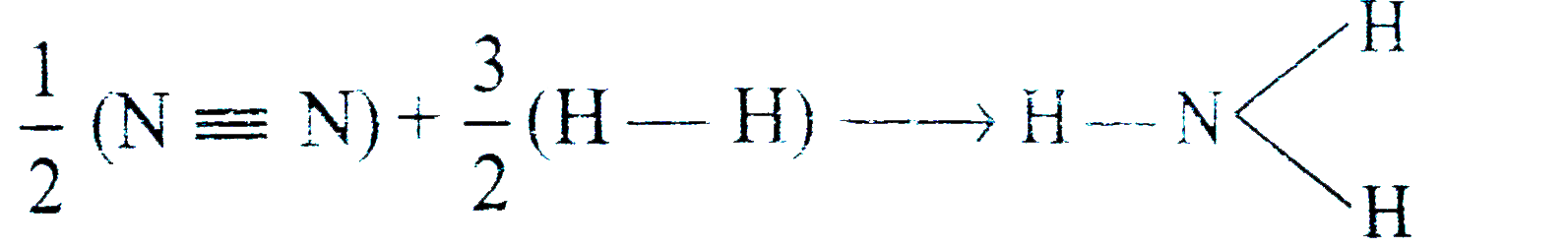A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Set 2|18 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level B|56 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Practice problems|81 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Objective Question Level-A
- On the basis of the following thermochemical data : (Delta(f)G^(@)H((a...
Text Solution
|
- The Habar's process of production of ammonia involves the equilibrium:...
Text Solution
|
- In which reaction there will be increase in entropy ?
Text Solution
|
- The species which by definition has zero standard molar enthalpy of fo...
Text Solution
|
- The standard enthalpy of formation of NH(3) is -46.0KJ mol^(-1) . If t...
Text Solution
|
- A 1.0g sample of substance A at 100^(@)C is added to 100 mL of H(2)O ...
Text Solution
|
- The entropy change involved in the isothermal reversible expansion of ...
Text Solution
|
- The incorrect expression among the following is
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- The approxiamte standard enthalpies of formation of methanol and octan...
Text Solution
|
- The sysmbols F,H,S,V(m) and E^(@) denote Helmholtz free energy, enthal...
Text Solution
|
- A piston filled with 0.04 mole of an ideal gas expands reversible from...
Text Solution
|
- For the complete combustion of ethanol, C(2)H(5)OH(l)+3O(2)(g)rarr2CO(...
Text Solution
|
- The standed free energy of fromation of NO(g) is 86.6 kj/ mol at 298...
Text Solution
|
- The enthalpies of combustion of carbon and carbon monoxide are -393.5 ...
Text Solution
|
- deltaU is equal to
Text Solution
|
- Given C(("graphite"))+O(2)(g)toCO(2)(g), Delta(r)H^(0)=-393.5kJ" "mo...
Text Solution
|
- Which of the following lines correctly show the temperatrue dependence...
Text Solution
|
- The combustion of benzene (l) gives CO(2)(g) and H(2)O(l). Given that ...
Text Solution
|
- If 1.5 L of an ideal gas at a apressure of 20 atm expands isothermally...
Text Solution
|