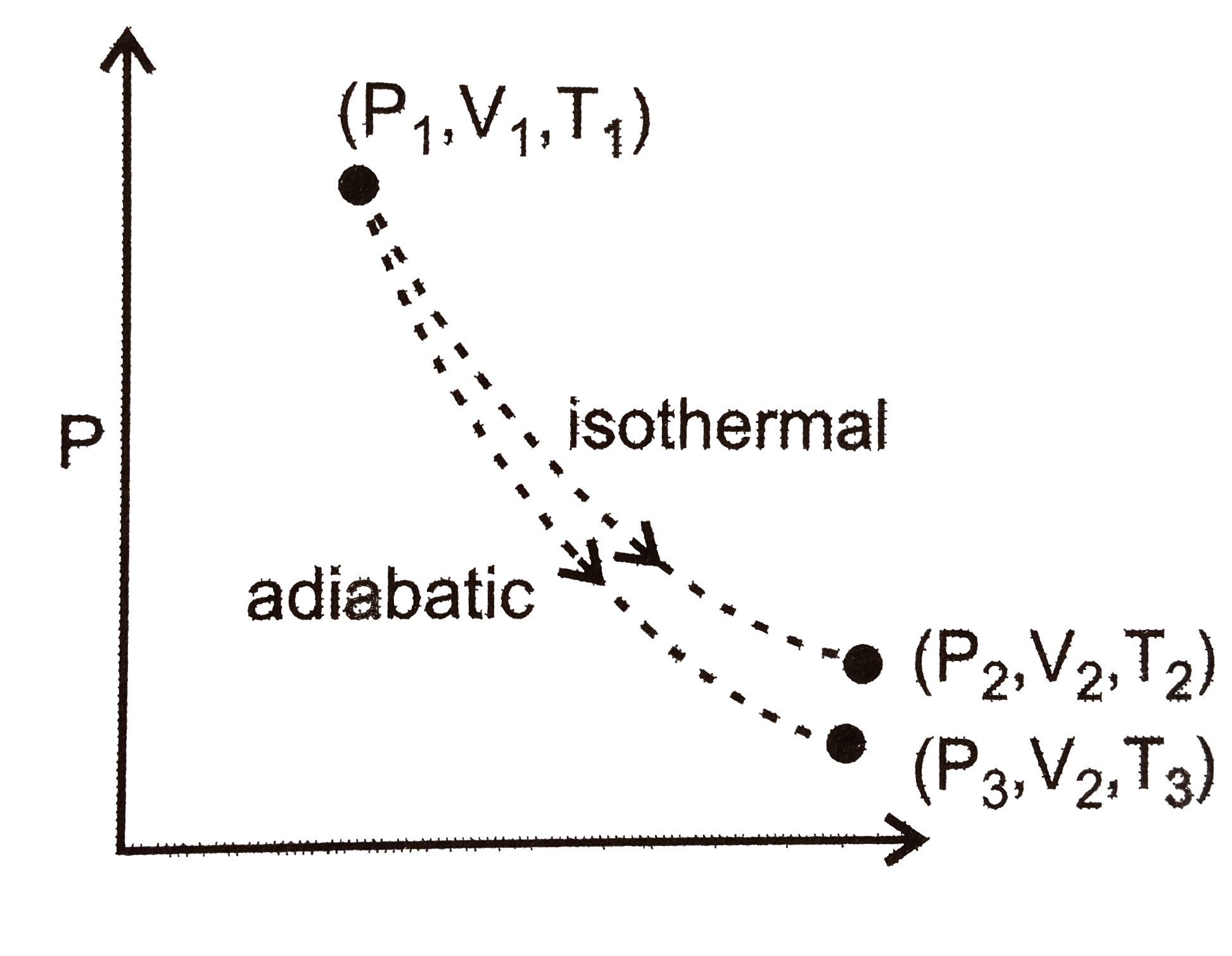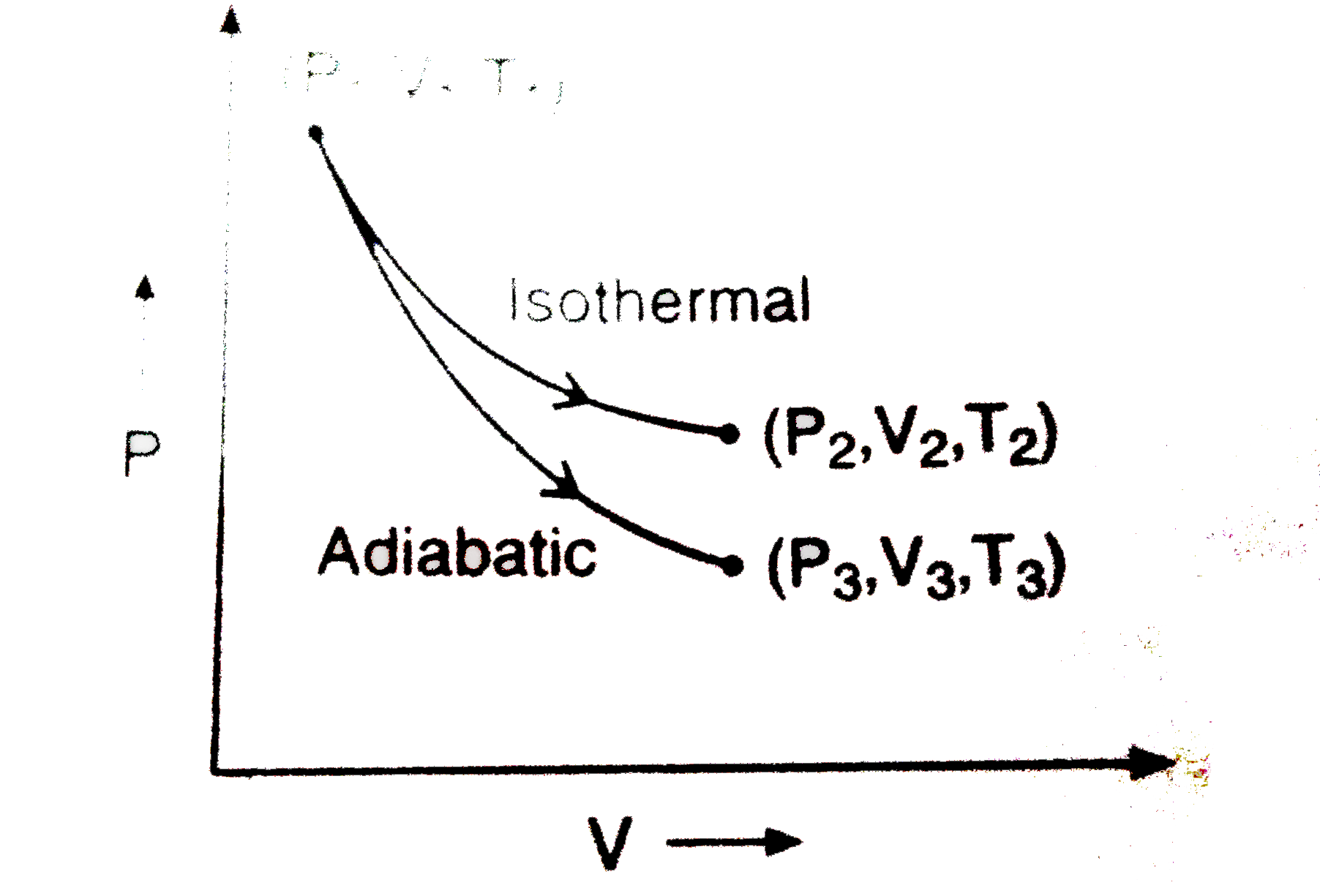A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level B|56 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level B Set II|8 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level-A|177 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Set 2
- Which is intensive property ?
Text Solution
|
- Which is an irreversible process?
Text Solution
|
- One mole of anahydrous MgCl(2) dissolves in water and liberates 25 cal...
Text Solution
|
- Following enthalpy changes are given: alpha-Dglucose (s) rarr alpha...
Text Solution
|
- If x and y are arbitrary extensive variables, then
Text Solution
|
- If x and y are arbitrary intensive variables, then
Text Solution
|
- H(2)O(g)+(1)/(2)O(2)(g)toH(2)O(g)," "DeltaH=x H(2)(g)+(1)/(2)O(2)...
Text Solution
|
- Which is correct about DeltaG?
Text Solution
|
- Dissociation of sodium azide is given below: NaN(3)toNa+3//2N(2), ...
Text Solution
|
- The lattice energy of KCl is 202 kcal/mo. When KCl is dissolved in wat...
Text Solution
|
- Which is a correct relationship?
Text Solution
|
- The standard Gibb's free energy change, DeltaG^(@) is related to equil...
Text Solution
|
- For the two equations given below: H(2)(g)+1//2O(2)(g)toH(2)O(l)+x(1...
Text Solution
|
- DeltaE=0, for which process :-
Text Solution
|
- For a reaction to be spontaneous in neither direction, which of the f...
Text Solution
|
- Enthalpy of neutralization of a strong acid by strong base: (1) has ...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|