A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level B Set II|8 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Assertion Reason Type Question|29 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Set 2|18 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Objective Question Level B
- For the process NH(3)(g) +HCI(g) rarr NH(4)CI(s)
Text Solution
|
- A gas is allowed to expand reversibly under adiabatic conditions. What...
Text Solution
|
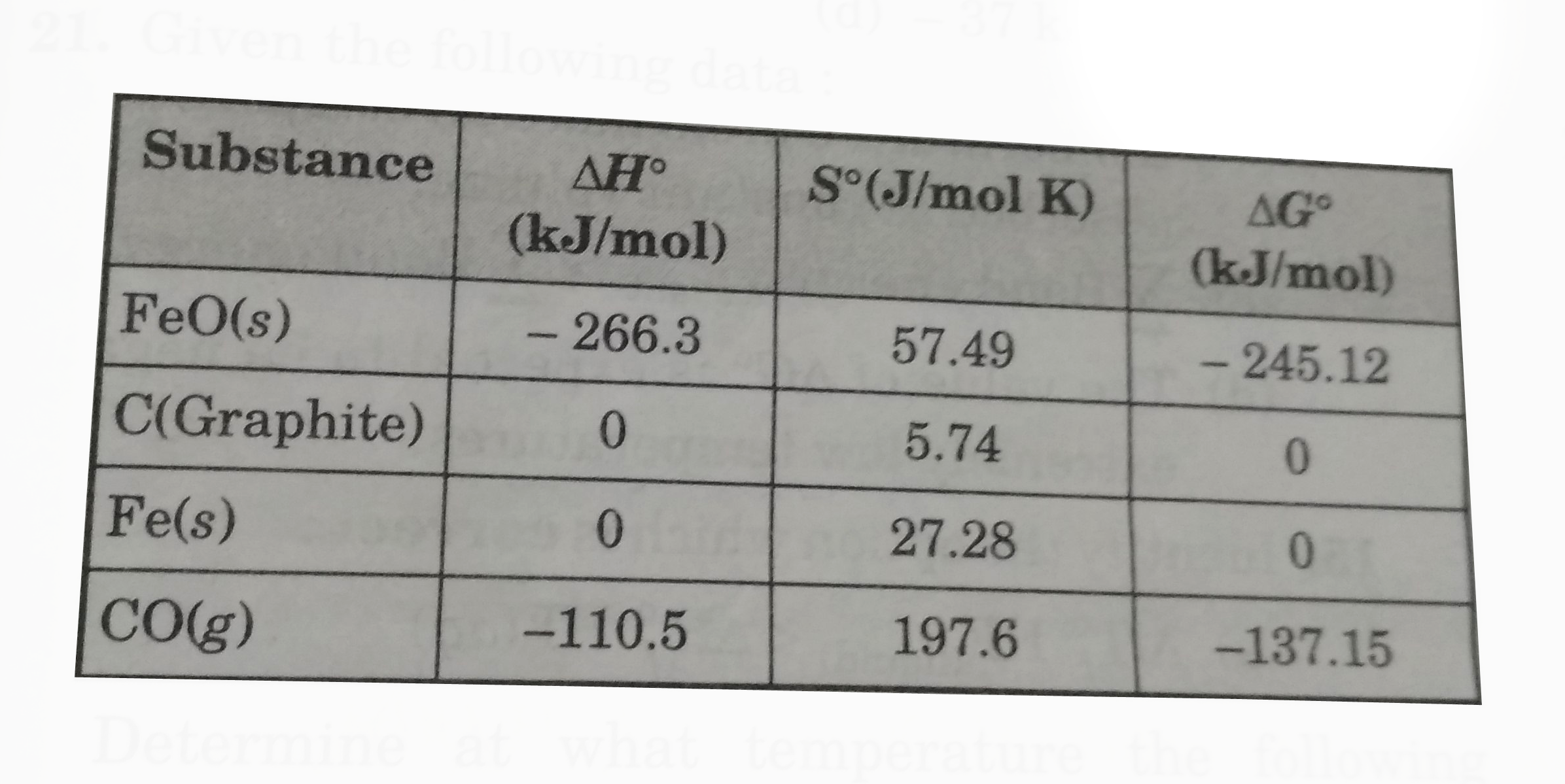
- Given the following data : Determine at what temperature the foll...
Text Solution
|
- Which of the following equations has/have enthalpy changes equal to De...
Text Solution
|
- The enthalpy change of which reaction corresponds to DeltaH(f)^(@) ...
Text Solution
|
- Enthalpy is equal to
Text Solution
|
- When a bomb calorimeter is sued to determine the heat of reaction, whi...
Text Solution
|
- For the reaction shown , which which is closest to the value o fDelta...
Text Solution
|
- An ice cube at 0.00^(@)C is placed is 200g of distilled water at 25^(@...
Text Solution
|
- Which reaction occurs with the greatest increase in entropy?
Text Solution
|
- The table given below lists the bond dissociation energy (E("diss")) f...
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown in P-V diagram. T...
Text Solution
|
- One gram mole of graphite and diamond were burnt to form CO(2) gas: ...
Text Solution
|
- Which among the following is not an exact differential?
Text Solution
|
- A gas expands adiabatically at constant pressure such that: T prop(1...
Text Solution
|
- 2Zn+O(2)to2ZnO," "DeltaG^(@)=-606J . . . (i) 2Zn+2Sto2ZnS," "D...
Text Solution
|
- The efficiency of the reversible cycle shown in the given figure is
Text Solution
|
- IN Haber's process of manufacturing of ammonia : N(2)(g)+3H(2)(g)to2...
Text Solution
|
- Consider the following statements: I. Change in enthalpy is always s...
Text Solution
|
- In C(2)H(4) energies of formation of (C=C) and (C-C) are -145 kJ/mol a...
Text Solution
|