A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Assertion Reason Type Question|29 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Assertion Reason Type Question Set-2|1 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Objective Question Level B|56 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Objective Question Level B Set II
- Which of the following are correct about irreversible isothermal expan...
Text Solution
|
- In adiabatic process, the work involved during expansion or compressio...
Text Solution
|
- For an ideal gas (C(p,m))/(C(v,m))=gamma. The molecular mass of the ga...
Text Solution
|
- An ideal gas in thermally insulated vessel at internal (pressure)=P(1)...
Text Solution
|
- For a reaction taking place in a container in equilibrium with its sur...
Text Solution
|
- An ideal gas is expanded from (p(1),V(1),T(1)) to (p(2),V2,T(2)) under...
Text Solution
|
- A reversible cyclic process for an ideal gas is shown below. Here, P, ...
Text Solution
|
- For a reaction, A hArr P, the plots of [A[ and [P] with time at temper...
Text Solution
|
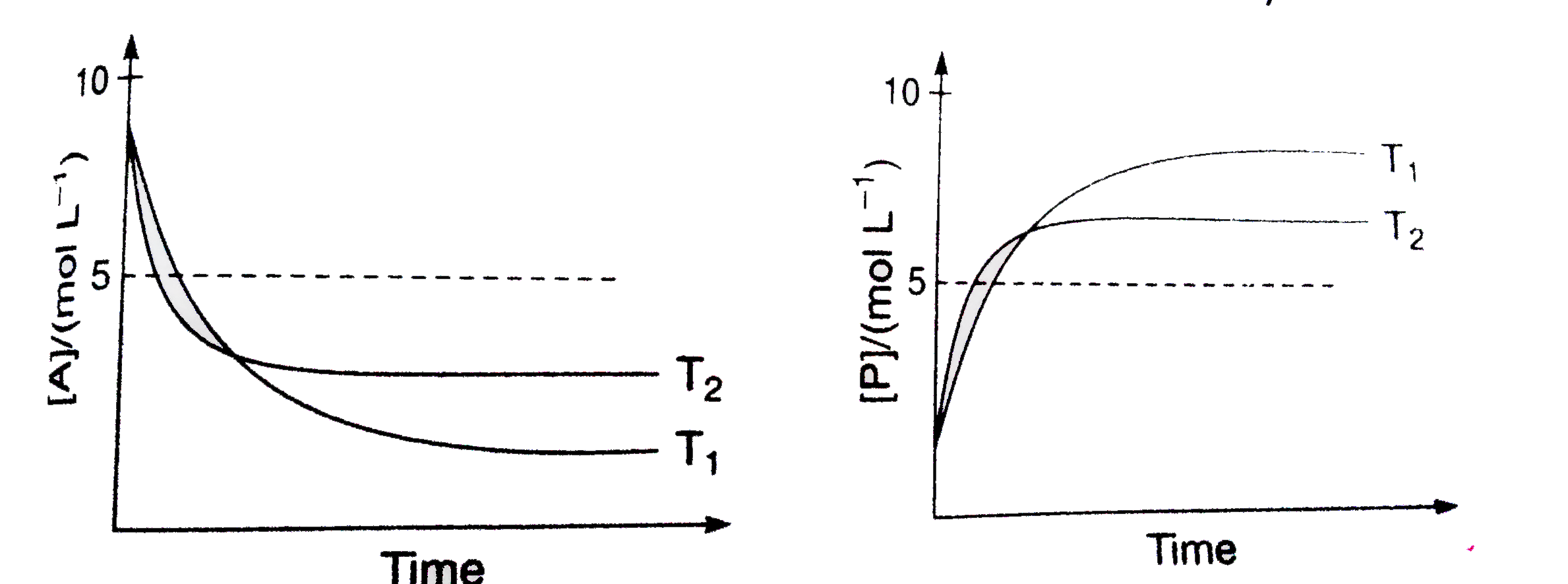 .
.