Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Linked Comprehension Type Questions|40 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment|10 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Matric Matching Type Questions|3 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Integer Type
- A bubble of 8 moles of helium is submerged at certain depth n water. T...
Text Solution
|
- For liquid enthalpy of fusion is 1.435 kcal mol^(-1) and molar entropy...
Text Solution
|
- For the reaction, Ag(2)O(s)hArr2Ag(s)+(1)/(2)O(2)(g) DeltaH,DeltaS a...
Text Solution
|
- Standard Gibbs free enegry change DeltaG^(Theta) for a reaction is zer...
Text Solution
|
- DeltaG^(ɵ) for the reaction X+YhArrC is -4.606 kcal at 1000 K. The equ...
Text Solution
|
- 4.48 L of an ideal gas at STP requires 12 cal to raise its temperature...
Text Solution
|
- In the present graph, the areas of circles A and B are 25 unit and 20 ...
Text Solution
|
- For the reaction, N(2)(g)+3H(2)(g)to2NH(3)(g) Heat of reaction at ...
Text Solution
|
- Gas (A(x)) has the ratio of cpecific heat, equal to 1.66. The value of...
Text Solution
|
- For a liquid the vapour pressure is given by: log(10)P=(-400)/(T)+10...
Text Solution
|
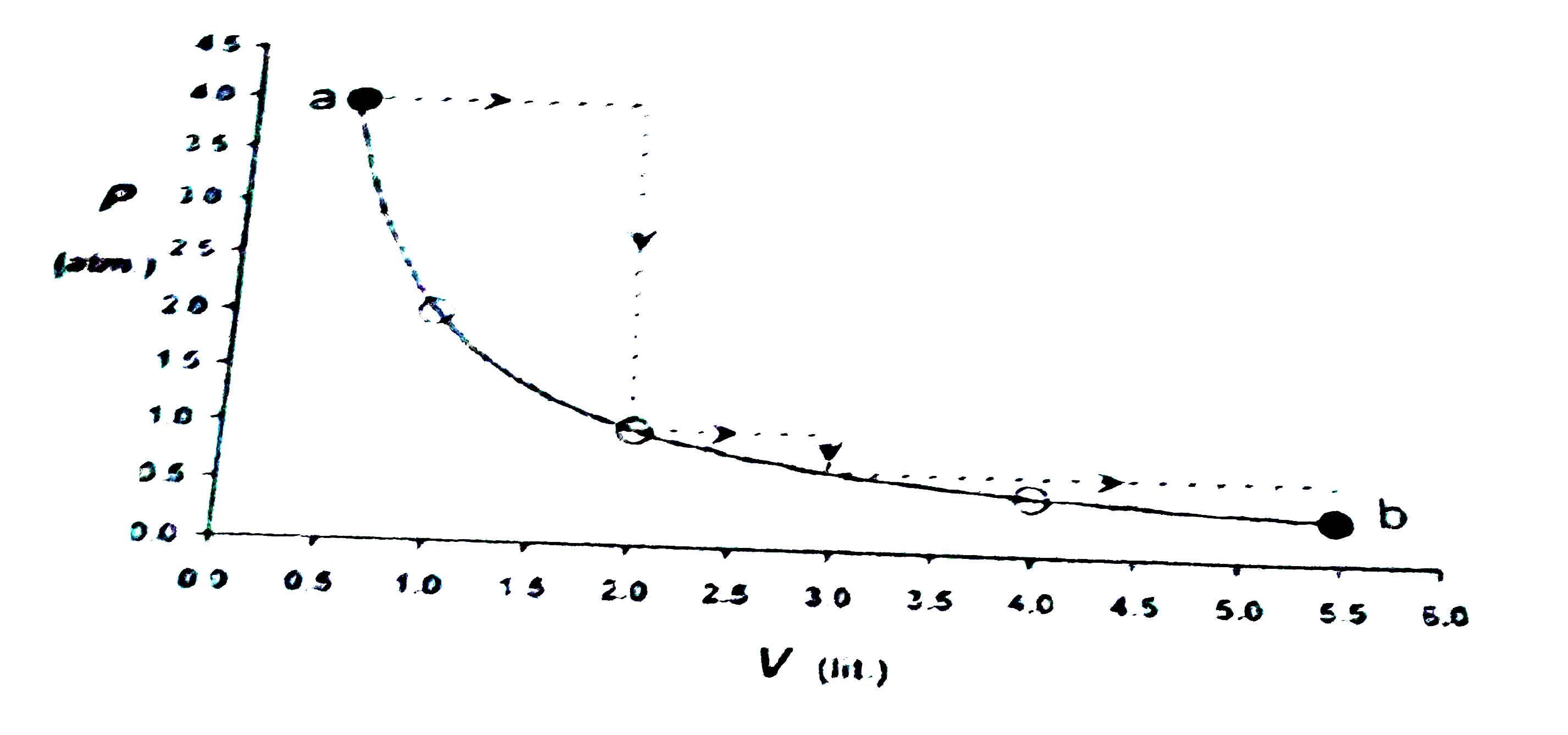
- One mole of an ideal gas is taken from a to b along two paths denoted ...
Text Solution
|