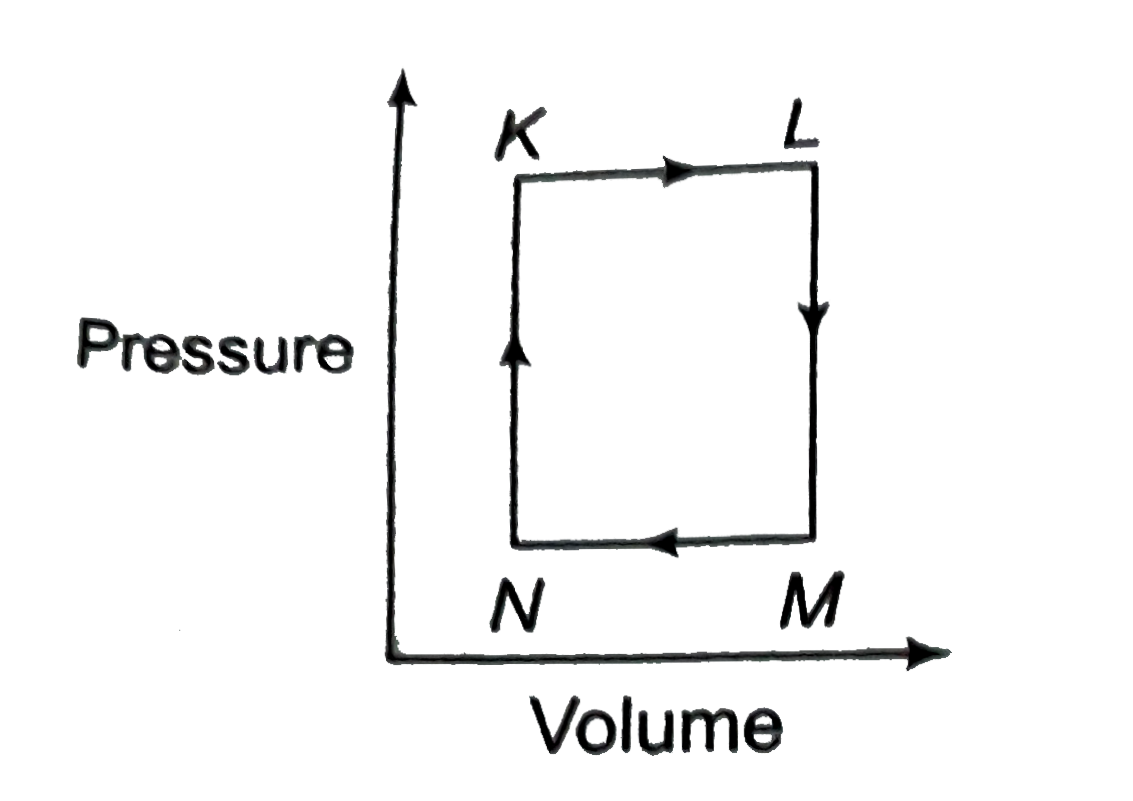
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment|10 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Self Assessment (Multiple Choice)|8 VideosChemical Thermodynamics and Thermochemistry
OP TANDON|Exercise Integer Type|11 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
OP TANDON|Exercise INTEGER|2 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-Chemical Thermodynamics and Thermochemistry-Linked Comprehension Type Questions
- 3 moles of CO(2) gas expands isothermally against external pressure of...
Text Solution
|
- When 100mL of 1.0M HCl was mixed with 100 mL of 1.0 M NaOH in an insul...
Text Solution
|
- When 100mL of 1.0M HCl was mixed with 100 mL of 1.0 M NaOH in an insul...
Text Solution
|
- Process, AtoB represent:
Text Solution
|
- The pressure at C is
Text Solution
|
- Q. Work doen in the process C to A is:
Text Solution
|
- Q. The process which occurs in going from BtoC is:
Text Solution
|
- The pressures at A and B in the atmosphere are, respectively,
Text Solution
|
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
- The thermodynamic property that measuers the extent of molecular disor...
Text Solution
|
- The thermodynamic property that measuers the extent of molecular disor...
Text Solution
|
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
- The pressure-volume of various thermodynamic process is shown in graph...
Text Solution
|
- The pressure-volume of various thermodynamic process is shown in graph...
Text Solution
|
- The pressure-volume of various thermodynamic process is shown in graph...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- Work is the mode of transference of energy. If the system involves gas...
Text Solution
|
- A fixed mass m of a gas is subjected to transformation of state: K to ...
Text Solution
|
- A fixed mass m of a gas is subjected to transfromation of states from ...
Text Solution
|