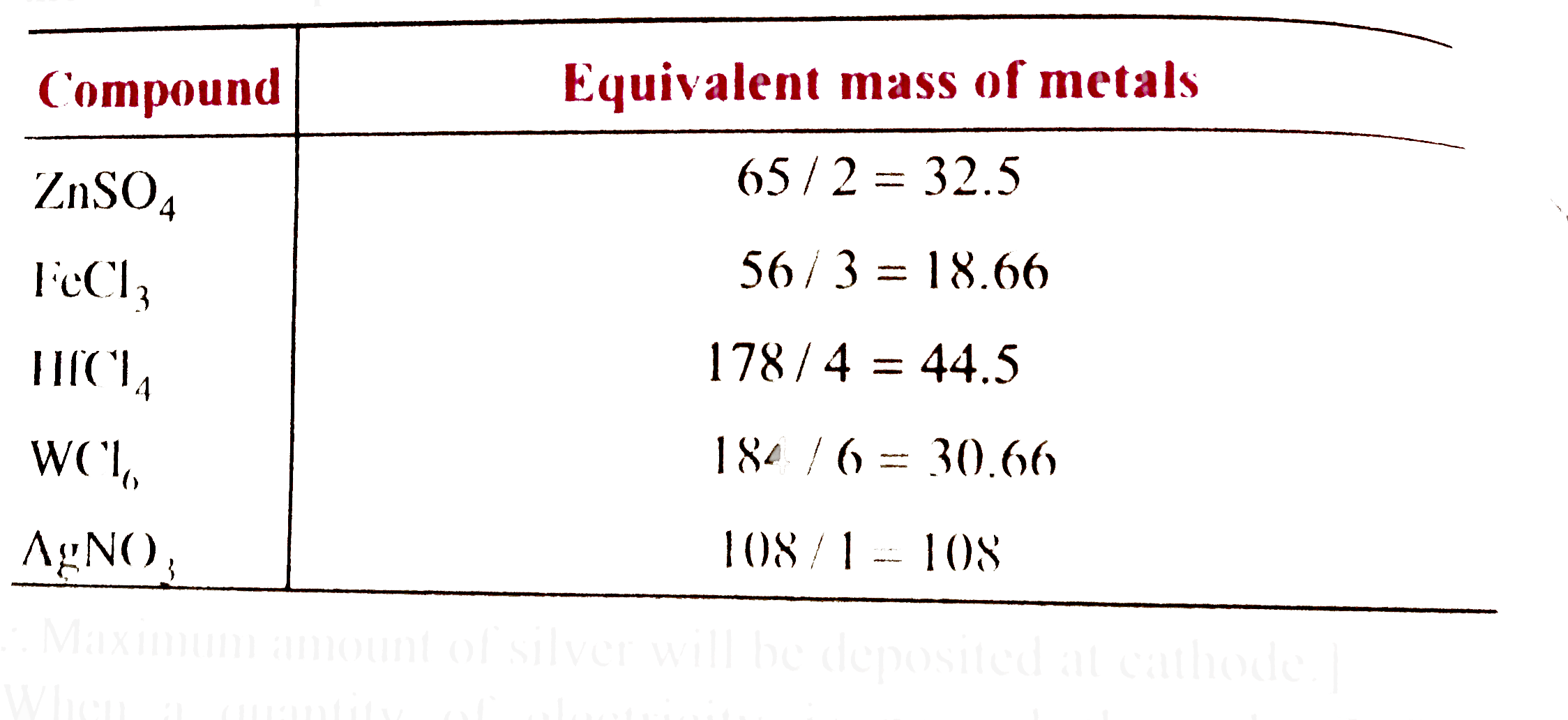A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
OP TANDON|Exercise PRACTICE PROBLEMS|94 VideosELECTROCHEMISTRY
OP TANDON|Exercise OBJECTIVE TYPE QUESTION (LEVEL -A)|194 VideosELECTROCHEMISTRY
OP TANDON|Exercise Matrix-Matching Type Question|3 VideosCOORDINATION COMPOUNDS
OP TANDON|Exercise SBJECTIVE TYPE|96 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Single Interger answer type questions|14 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-ELECTROCHEMISTRY-ILLUSTRATIONS
- Cost of electricity for the production of X litre H(2) at STP at catho...
Text Solution
|
- What current is to be passed for 0.25 sec for deposition of certain we...
Text Solution
|
- If the aqueous solutions of the following salts are electrolysed for 1...
Text Solution
|
- When a quantity of electricity is passed through CuSO(4) solution, 0.1...
Text Solution
|
- Number of faraday's required to generate one gram atom of magnesiu fro...
Text Solution
|
- A direct current deposits deposits 54 g of silver (Atomic mass = 108) ...
Text Solution
|
- The charge required for the reduction of 1 mol Cr(2)O(7)^(2-) ions to ...
Text Solution
|
- The equivalent conductance of 1 M benzoic acid is 12.8 ohm^(-1) cm^(2)...
Text Solution
|
- Equivalent conductances of NaCl, HCl and CH(3)COONa at infinite diluti...
Text Solution
|
- The specific conductance of saturated solution of AgCl is found to be ...
Text Solution
|
- The specific conductivity of N/10 KCl solution at 20^(@)C is 0.0212 oh...
Text Solution
|
- The resistance of 1N solution of acetic acid is 250ohm, when measured ...
Text Solution
|
- Resistance of a conductvity cell filled with a solution of an electrol...
Text Solution
|
- If the molar conductance values of Ca^(2+) and Cl^(-) at infinite dilu...
Text Solution
|
- The molar conductivities of KCl, NaCl and KNO(3) are 152,128 and 111" ...
Text Solution
|
- At a particular temperature, the ratio of molar conductance to specifi...
Text Solution
|
- The oxidation potential of a hydrogen electrode at pH = 10 and P(H(2))...
Text Solution
|
- The value of equilibrium constant for a feasible cell reaction is :
Text Solution
|
- E^(@) for the electrochemical cell Zn(s)|Zn^(2+) 1 M (Aq.)||Cu^(2+) ...
Text Solution
|
- The value of the reaction quotient, Q for the following cell is Zn(s...
Text Solution
|